Tungsten Disulfide Property
- Details
- Category: Tungsten Information
- Published on Wednesday, 19 June 2019 21:11
Tungsten disulfide property is diamagnetic, lubricious and easy to be dissociative. It is a compound of tungsten and sulfur, chemical formula is WS2, molecular weight 247.97, the state is black gray powder, appears in the natural world as a tungstenite. The relative density is 7.510. It adopts a layered structure related to MoS2, with W atoms situated in trigonal prismatic coordination sphere.
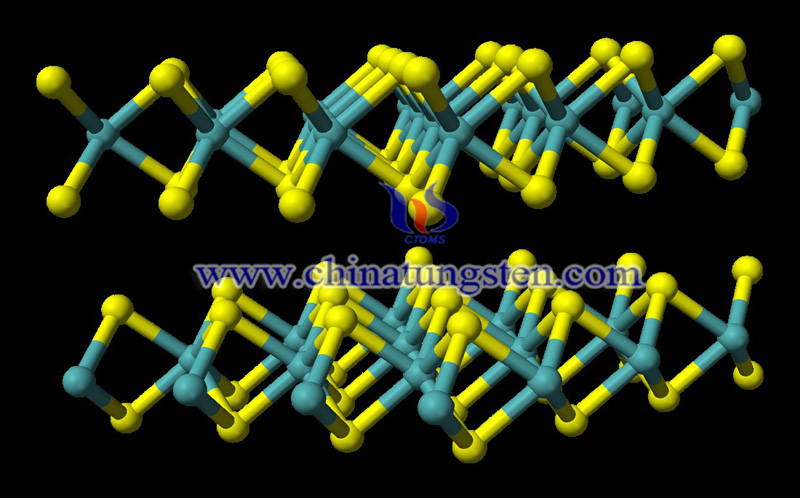
Tungsten disulfide (WS2) is a layered transition metal dichalcogenide with a reported band gap of 1.8 eV in bulk and 1.32-1.4 eV in its thin film form. 2D atomic layers of metal dichalcogenides have shown changes in conductivity with applied electric field. This makes them an interesting option for channel material in field effect transistors. Tungsten Disulfide is a member of the family of materials known as transition metal dichalcogenides (TMDs). These materials are much like graphene in their nature, being 2-D materials that have property differing significantly from those of their bulk properties. Thanks to its unique property, WS2 is currently one of the most studied TMDs in literature.
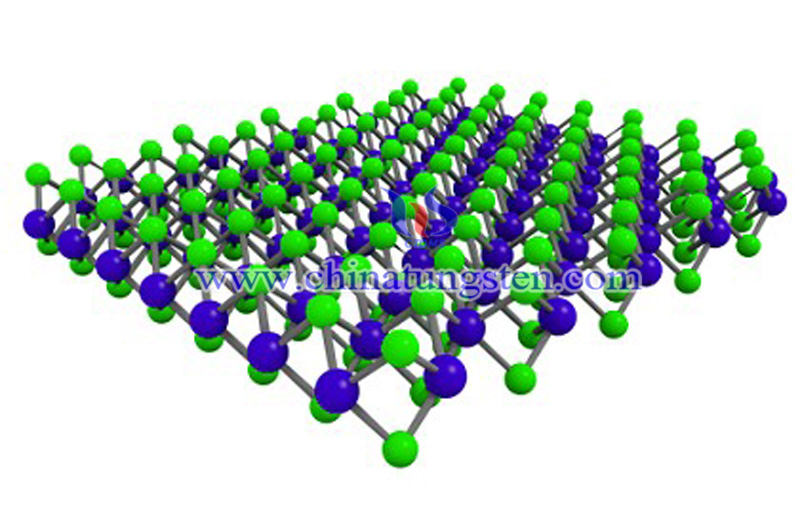 WS2 is slightly soluble in cold water and soluble in hot water. But it does not dissolve in hydrochloric acid and alkali. It is soluble in molten alkali and insoluble in alcohol and can react with hot concentrated sulphuric acid, nitric acid, aqua regia and other strong oxidants, and can be converted into WO3 by heating in air or oxygen. When heated to 1250 ° C in a vacuum, it decomposes into tungsten and sulfur. The mixture of tungsten trisulfide and sulfur was co-heated to 900 ° C in a stream of dry pure nitrogen, and the residue of it was tungsten disulfide.
WS2 is slightly soluble in cold water and soluble in hot water. But it does not dissolve in hydrochloric acid and alkali. It is soluble in molten alkali and insoluble in alcohol and can react with hot concentrated sulphuric acid, nitric acid, aqua regia and other strong oxidants, and can be converted into WO3 by heating in air or oxygen. When heated to 1250 ° C in a vacuum, it decomposes into tungsten and sulfur. The mixture of tungsten trisulfide and sulfur was co-heated to 900 ° C in a stream of dry pure nitrogen, and the residue of it was tungsten disulfide.
Scientists began to study on the property of tungsten disulfide flakes after the discovery of simple mechanical exfoliation techniques to isolate single layers of 2-D materials. It was shown that, just like MoS2, the band-gap of tungsten disulfide change from an indirect band-gap of 1.4eV to a direct band-gap of 2eV when changing from a bulk material to a 2-D material. Due to its band-gap, WS2 is seen as a significantly interesting material for many areas of application.
Just like MoS2, WS2 possesses a high on and off ratio in field-effect transistors, controllable spin and valley polarisation, strong geometrical confinement of excitons, and tunable photoluminescence. Additionally, WS2 could lead to increased interest in areas such as photodetectors and multi-junction photovoltaics.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com