Phosphorus Removal Process Before Ion Exchange
- Details
- Category: Tungsten Information
- Published on Saturday, 20 April 2019 15:54
With the shortage of mineral resources, the national environmental protection policy has become increasingly stringent, and attention has been paid to the upgrading of scheelite smelting methods. The development and application of tungsten smelting processes with high metal yield and low energy consumption, which can achieve closed circuit recycling and zero emission, can not only create profits for enterprises, but also preserve wealth for future generations.
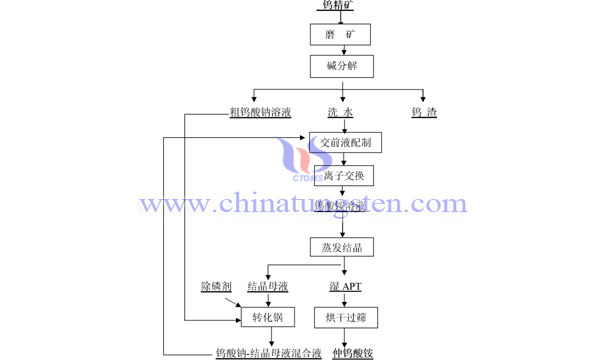
In order to prevent the formation of Mg(OH)2, the classical magnesium salt dephosphorization process needs to neutralize sodium tungstate solution with hydrochloric acid to pH < 12 before adding magnesium chloride. Because the introduction of chloride ions will seriously affect the subsequent ion exchange tungsten adsorption, this method is difficult to meet the process requirements. Researchers believe that the crude sodium tungstate solution and crystalline mother liquor are heated in the converter, and the dephosphorization agent calcium carbonate is added to control the vapor pressure in the range of 0.1 MPa to 0.3 MPa. After fully reacting under alkaline conditions, the mixture of sodium tungstate solution and crystalline mother liquor and calcium phosphate precipitate are separated by plate-frame filter press. The process of phosphorus removal before ion exchange is as follows:
Under the conditions of 150 ml crude sodium tungstate solution containing WO3 150 g/L and P 1 g/L and 120 min reaction time, 2.5 times theoretical dosage of calcium carbonate dephosphorizing agent and 1.57 mol/L OH concentration of solution, the residual phosphorus concentration in solution was 0.038 g/l, 0.015 g/l, 0.006 g/l and 0.005 g/l respectively, and the dephosphorization rate was 0.038 g/l, 50 ℃, 65 ℃ and 80 ℃ heated by steam. The phosphorus removal efficiency is 95.57%, 98.13%, 99.26% and 99.38% respectively. The phosphorus removal efficiency meets the requirements of industrial production process.
The process of removing phosphorus before ion exchange has the following advantages: 1. After removing phosphorus from sodium tungstate solution-crystalline mother liquor mixture, the P content in solution can reach <0.06g/L, so the amount of phosphate added during decomposition of tungsten concentrate can exceed 1.0 times of theoretical amount, the whole process is more flexible, the decomposition rate of valuable metal tungsten is effectively improved, the purity is higher, and the total recovery rate of tungsten is up to 95%, the highest phosphorus removal rate can reach 99.40%.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com