Bismuth Trioxide - Bismuth Tungstate Heterojunction Photocatalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 02 April 2019 22:48
Bi2WO6, as a kind of p-type semiconductor material, has perovskite lamellar structure. Its band gap is about 2.7eV. Bi2WO6 has strong absorption in the visible region with wavelength greater than 420nm. It can use both ultraviolet and visible light in sunlight. It is more and more popular among researchers. However, due to poor adsorption, the separation efficiency of photogenerated carriers is low.
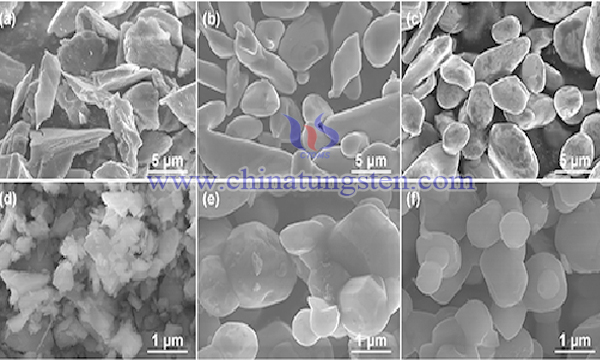
In order to improve the existing technology and improve the production efficiency, bismuth nitrate and ammonium metatungstate were used as raw materials to synthesize bismuth trioxide-bismuth tungstate heterojunction photocatalyst by solvothermal method in one step. The specific operation process is as follows:
(1)dissolve (NH4)10W12O41·5H2O in ethylene glycol under the condition of magnetic stirring, and record it as solution A.
(2)Bi(NO3)3·5H2O with 26.4 - 36 times the molar weight of (NH4)10W12O41·5H2O as described in step (1) was dissolved in the medium amount of ethylene glycol in step (1) with the assistance of ultrasound, and was recorded as solution B.
(3)Under the condition of magnetic stirring, solution A is slowly added to solution B, followed by ethanol with a volume of 2-3 times that of ethylene glycol as described in step (1). Continuous stirring for 10 minutes until the mixture is uniform, which is recorded as solution ℃.
(4)Transfer C solution into hydrothermal reactor and place it in blast dryer at 160-200 ℃ for 5-12h at constant temperature.
(5)Remove the reactant, wash it several times with absolute ethanol, and dry it for 6 hours at 60 ℃.
(6)Bismuth trioxide-bismuth tungstate heterojunction photocatalyst was prepared by calcining the dried product in a muffle furnace at 350-450 ℃ and grinding it after cooling.
Compared with Bi2WO6, the prepared bismuth trioxide-bismuth tungstate heterojunction photocatalyst has higher photocatalytic efficiency, more than 95% degradation rate and better stability. The reason is that Bi2O3/Bi2WO6 has a typical p-n heterostructure. Under light conditions, the electrons in the valence bands of Bi2WO6 and Bi2O3 are stimulated to transit to the conduction band. Because the conduction band position of Bi2O3 is more negative than that of Bi2WO6, the electrons on the conduction band of Bi2O3 will migrate to the layer structure of Bi2WO6 rather than accumulate on the surface, thus realizing photogenerated electrons and photosynthesis. With the separation of holes, the heterostructures satisfy the characteristics of high activity and wide spectral response.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com