Ammonium Paratungstate Formation Mechanism
- Details
- Category: Tungsten Information
- Published on Wednesday, 31 January 2018 17:13
Ammonium paratungstate formation mechanism is due to the tungstate in the form of tungstate tungsten ions in solution, when the solution is acidified, the tungstate will react with hydrogen ions to produce a variety of polyoxometalates of tungsten ions. The reaction can be expressed as:
n WO42−+ p H+= [Hx Wn O4n-0.5(p-x)] (2n-p)-+ 0.5(p-x) H2O

Ammonium paratungstate formation mechanism is mainly the role of polymerization, and the formation of polymerization components mainly with the solution pH. However, the solution temperature, type, concentration, time and other factors on the formation of ammonium paratungstate also have a certain impact. The reaction of tungstate to form different isopolyacid ions under different pH conditions is the following equation:
The formation of ammonium paratungstate reaction mechanism solution pH of about 6, the first tungstate ions quickly react with hydrogen ions to produce HW6.

- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com


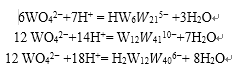

 sales@chinatungsten.com
sales@chinatungsten.com