Sodium Tungstate Physical and Chemical Properties
- Details
- Category: Tungsten Information
- Published on Tuesday, 06 February 2018 13:04
Sodium tungstate is a chemical that is soluble in water, sparingly soluble in hydrogen but insoluble in ethanol, and glossy, lamellar or crystalline powders. Sodium tungstate weathering when exposed to the air, when dissolved in water, the aqueous solution was slightly alkaline, heated to 100 ℃ will lose crystal water and become anhydrous.
|
Chinese Name |
Sodium Tungstate |
Water-Soluble |
Alkaline |
|
English Name |
Sodium Tungsten Dihydrate |
Density |
3.23-3.25 |
|
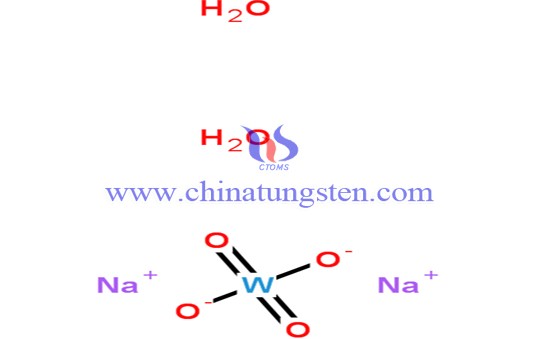
Chemical Formula |
Na2WO4·2H2O |
Exterior |
Colorless Crystal Or White Crystalline Powder |
|
Molecular Weight |
329.86 |
Application |
Manufacture Of Metal Tungsten, Tungstic Acid, Tungstate |
|
Melting Point |
698℃ |
Dangerous |
Avoid Contact with The Human Body. |
|
Level Classification |
Ordinary Level, High-Grade |
Storage and Transportation Characteristics |
Store in A Cool, Dry, Well-Ventilated Place Away from Incompatible Materials. |
Reacts with strong acids (except hydrofluoric acid) such as hydrochloric acid and sulfuric acid to form yellow tungstic acid which is insoluble in water, but reacts with phosphoric acid or phosphate to form phosphotungstic acid complex with tartaric acid, citric acid, oxalic acid and other organic acids react to form the corresponding organic acid complexes.
Preparation of sodium tungstate is based on the reaction of tungsten oxide and sodium hydroxide, or tungsten concentrate and sodium hydroxide pressure cook, generate sodium tungstate solution, Through refining, filtration, ion exchange and other processes, the separation of impurities, and finally by evaporation of sodium tungstate products.
- AMT Manufacturer & Supplier, Chinatungsten Online: ammonium-metatungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com