Synthesis of V2O5-WO3/Tio2 Catalysts from Ammonium Paratungstate in SCR Applications
- Details
- Category: Tungsten Information
- Published on Saturday, 26 June 2021 00:54
In urea-SCR of passenger Diesel vehicles, the NOx reduction step of urea-SCR is usually catalyzed by redox zeolites. Technology for stationary sources r emains to be dominated by catalysts that contain V2O5 and WO3 supported on TiO2 (anatase). They are meanwhile also applied for mobile sources, in heavy utility vehicles, where they are adapted to the different targets. Thus, V2O5-WO3/TiO2 catalysts had been synthesized from ammonium paratungstate (APT) for SCR applications.

The synthesis method of V2O5-WO3/TiO2 catalysts is as following steps: Sources of W and V were ammonium paratungstate ((NH4)10(H2W12O42)·4H2O, APT) and ammonium metavanadate (NH4VO3, 99%) were used as W and V source respectively.
WO3 was deposited on the TiO2 hydrate by wet impregnation of APT from its aqueous solution, the concentration of which was chosen to obtain a WO3 loading of 10.0 wt-%. The material was dried at 383 K overnight and calcined in flowing synthetic air (20% O2/He) at 713 K for 1 h, which brought the BET surface area down to 142 m2/g. This material was wet impregnated with an aqueous NH4VO3 solution to obtain a loading of 1.5 wt-% V2O5, followed by overnight drying at 383 K. The resulting catalyst with a W/V atomic ratio of 2.6, has been labelled V1.5W10. Transition metal coverages can be evaluated using known data for areal densities in the monolayer.
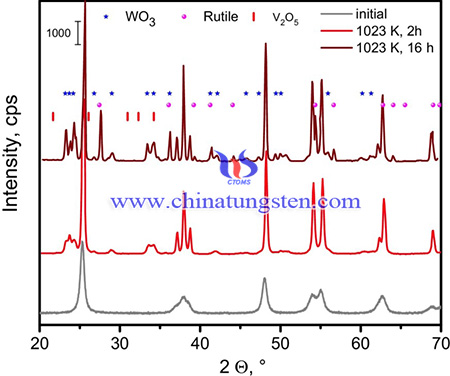
In summary, V2O5-WO3/TiO2 catalysts are synthesized from ammonium paratungstate (APT) for SCR applications. The catalysts possess high catalytic activity and is feasible for the application of NOx sensors. The catalyst containing 1.5 wt-% V2O5 and 10 wt-% WO3 on anatase of initially 140 m2/g, these ranges were ≈42 m2/g and ≈20 m2/g, segregation of tungstate results in formation of highly active V-O-V structures from less active isolated vanadate species previously separated by excessive amounts of tungstate. In this notion, the role of tungstate is to provide optimum sizes of vanadate ensembles. An experimental strategy is proposed which will allow deciding if there is an additional favorable tungstate influence on the vanadate sites or if the activity loss after the second maximum results from decreasing intrinsic activity of larger vanadate islands.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com