Nanoscale Catalyst for Mercury Removal Produced from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Monday, 25 January 2021 02:10
Mercury pollution, serving as a toxic heavy metal, removal of mercury has always been an important environmental issue. As the largest mercury emissions country, Chinese mercury emissions derived from coal-fired industry increased from 45.1 t in 1978 to 175.7 t in 2014. It was estimated that atmospheric mercury emissions derived from coal incineration were 202.3 tons in 2018.
Mercury derived from coal-fired flue gas had three forms: oxidized mercury (Hg2+), elemental mercury (Hg°) and particle-bound mercury (HgP). The 99% HgP and 80% Hg2+ could easily be collected via dust collection devices and WFGD systems, respectively. But these techniques were difficult to capture Hg° because of its volatility and insolubility. Therefore, Hg0 oxidation was the key step for the synergistic controlling technology. Cerium oxide showed excellent activity and oxygen storage ability because of its multiple valence states (Ce4+/Ce3+). It was found that Mercury Oxidation Efficiency (MOE) of CeO2-based catalyst was 80–100% in presence of HCl and NO. However, the SO2 presence and hydrothermal treatment decreased the MOE to below 50%.
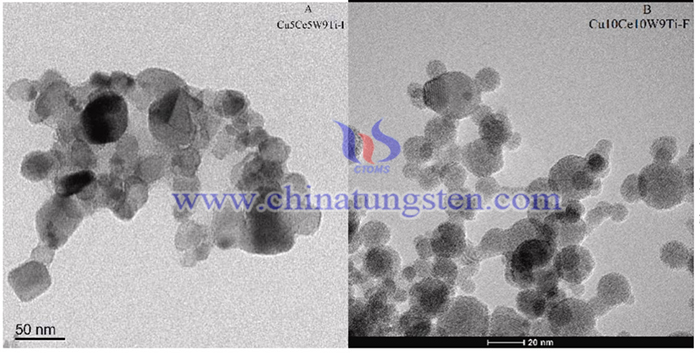
To overcome these drawbacks, a flame synthesis of Cu10Ce10W9Ti-F catalyst with the high temperature and hydrothermal resistance has been conducted. The Cu and Ce were selected as active reagents of catalyst since CuO-CeO2-WO3/TiO2 showed high Hg0 oxidation efficiency and superior SO2 resistance.
The flame synthesis of Cu10Ce10W9Ti-F catalyst can be concluded as below:
The 8:100 ratio of CH4 and air was used as fuel to mix with air to form premixed gas. The flow rate of the mixture gas was 20 L min−1, and the flame temperature was kept at about 1600 °C. The flame temperature was measured via the thermoelectric couple. The catalyst precursor passed through high-pressure (0.2 Mpa) air to form 100–200 nm aerosol in the atomizer and then entered the flame to form catalyst via heating, evaporation, burning and agglomeration. The precursors included C16H28O6Ti (disopropyl diacetylacetonate titanate, A.R.), C24H45CeO6 (cerium 2-ethylhexanoate, A.R.), W(CO)6 (hexacarbonyl tungsten, A.R.), C7H15COO)2Cu (copper 2-ethylhexanoate, A.R.) and solvent C4H4O (tetrahydrofuran, A.R.). The atomized air flow of the precursor is maintained at 2 L min−1. The liquid precursor flow rate was 10–15 ml h−1. The synthesized catalyst was collected on the stagnation plate (1 mm aluminum plate) under the thermophoretic force. The catalyst production rate was about 5 g h−1.
The wet impregnation synthesized catalysts were used as contrast samples of Flame made catalysts. Commercial TiO2 Degussa P25 served as the precursor. Cu5Ce5W9Ti-I catalysts was prepared by dispersal of a certain mass of TiO2 powder into Cu(NO3)2 (cupric nitrate hexahydrate, A.R.), Ce(NO3)3·6H2O (cerium nitrate hexahydrate, A.R.) and 5(NH4)2O·12WO3·5H2O (ammonium paratungstate, A.R.) solution to obtain Cu5Ce5W9T-I slurry. The slurries were treated by microwave for 15 min, stirred for 24 h, heated at 110 °C for 12 h and then calcined at 500 °C for 4 h in air. Then, the catalysts was ground and sieved to 40–60 mesh as contrast samples. The optimal percentage was selected according to the result of our previous study.
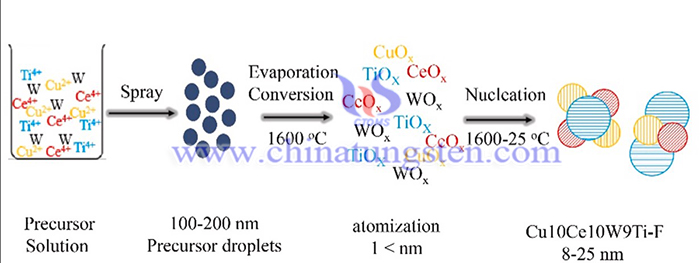
In summary, The flame synthesis method has successfully synthesized nanoscale Cu10Ce10W9Ti-F particles smaller lattice size of 8–25 nm, more stable carrier structure , more oxygen vacancies, and a high Hg0 oxidation efficiency (MOE) of 83.9–99.7% at 100–450 °C. These properties and structure served as key factors for the great hydrothermal stability on flame made catalysts. The excellent nanostructure increased the content of oxygen vacancies (such as Ce3+) and L-acid site (O2−), making for the thermostability of flame made catalysts. In addition, the carrier TiO2 of Cu10Ce10W9Ti-F existed in form of highly thermostable rutile rather than anatase with low thermostability on Cu5Ce5W9Ti-I. The high temperature (1600 °C) promoted the TiO2 transform to more stale crystal type in flame synthesis. High Hg0 oxidation efficiency (MOE) of 83.9–99.7% at 100–450 °C proved excellent oxidation activity of Cu10Ce10W9Ti-F catalyst. The thermal and hydrothermal treatment (700 °C) only decreased MOE by less than 5%.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com