BWO-OV/OCN Heterojunction Photocatalytic Material From Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2021 01:01
Recently, bismuth tungstate (BWO) with typical perovskite layered structure has been developed for photocatalytic application under visible light irradiation. Oxygen-enriched graphitic carbon nitride (OCN) OCN can generate H2O2 more easily owing to the oxygen-enriched structure and the black body nature enhance the light absorption capability. Thus, it is a potential material to be adopted to enhance the photocatalytic activity of BWO.
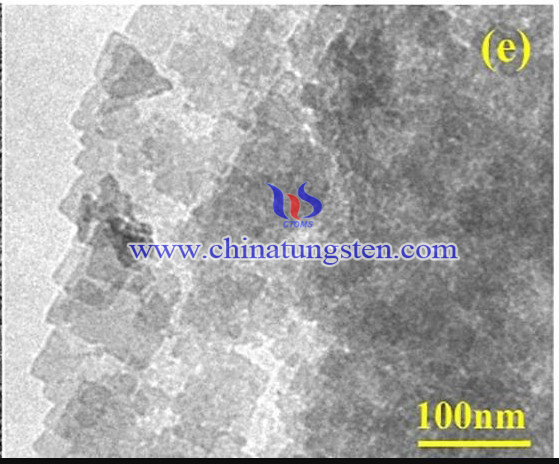
It was reported recently that a BWO-OV/OCN heterojunction photocatalytic material was synthesized by simple hydrothermal method. ammonium paratungstate (APT) has used to as a starting material. The preparation procedure can be concluded as below:
Octadecyltrimethylammonium chloride (OTAC), sodium tungstate dehydrate (Na2WO4·2H2O), bismuth nitrate (Bi(NO3)3·5H2O), dicyandiamide (C2H4N4), ammonium paratungstate (APT), Tetracycline (TC), isopropanol (IPA), Tetramethylpiperidinyloxy (TEMPO) and sodium oxalate (C2Na2O4) were used as precursor materials. The preparation was divided into two steps.
a. Preparation of OCN
OCN was synthesized via simple calcination by previous report [42]. Firstly, 4.204 g (50 mmol) of dicyandiamide and 1.521 g (0.5 mmol) of ammonium paratungstate were transferred into a lidded corundum crucible to mix thoroughly. Then the mixture was calcined at 500 °C for 4 h with the heating rate of 3 °C min−1. Finally, the product was obtained after cooling to room temperature.
b. Preparation of BWO-OV/OCN
0.1 g of Na2WO4·2H2O (0.3 mmol) and 0.04 g of OTAC (0.115 mmol) were poured into 40 mL of deionized water. After completely dissolved, the powder of about 0.243 g of Bi (NO3)3·5H2O (0.5 mmol) was added while being stirred on the multi-point magnetometer. After stirring for 1 h, 0.475 g the prepared OCN was added, and the new mixture was continued mixing overnight. The mixture was then transferred into teflon-lined high-pressure reactor (100 mL) and reacted in a 140 °C oven for 24 h. Finally, the obtained products after filtration were washed several times by deionized water and ethanol respectively. After drying at 60 °C for 12 h, the final 4BWO-OV/OCN composite was obtained. xBWO-OV/OCN (1: x in weight = OCN: BWO-OV in weight) heterojunction photocatalyst was obtained by adding different amount OCN. BWO-OV was prepared by the same procedure without adding OCN. The BWO-OV with different weight ratio in composite of 25 wt%, 50 wt%, 66 wt%, 75 wt%, 80 wt%, 83 wt% were prepared and signed as 0.33BWO-OV/OCN, 1BWO-OV/OCN, 2BWO-OV/OCN, 3BWO-OV/OCN, 4BWO-OV/OCN, 5BWO-OV/OCN, respectively.
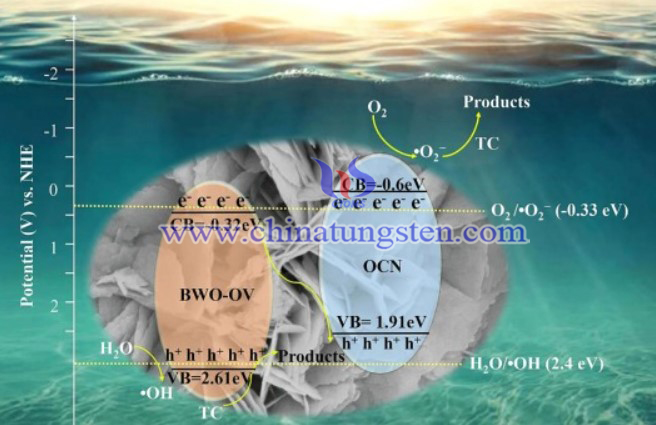
The structure and morphology of prepared samples were explored via SEM and TEM. The photoelectric property was also studied in detail. The photocatalytic activity of samples was investigated via TC degradation.
In conclusion, BWO-OV/OCN heterojunction was successfully prepared via facile hydrothermal process. The induced oxygen vacancies benefit the exposure of reaction sites, the black body nature and oxygen-rich structure of OCN would enhance the light absorption capability, and then the photocatalytic performance can be improved. provides a potential strategy to develop photocatalysts for pollutant elimination in practice.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com