Advantages of Tungsten Oxide-Carbon Composites
- Details
- Category: Tungsten's News
- Published on Wednesday, 08 February 2023 22:20
The introduction of carbon material into tungsten oxide to obtain tungsten oxide-carbon composites owns several advantages. One is that the composite material can integrate the advantages of tungsten oxide and carbon materials. Another is that it is more likely to form simple structures with high structural stability. Graphene is a flat monolayer based on a honeycomb lattice of single carbon atom layers.
This special two-dimensional structure gives it an ultra-high theoretical specific surface area (2675 m2 g-1) and provides high thermal and electronic conductivity. Zeng et al. synthesized layered sandwich composites consisting of WO3 nanosheets and graphene. The researchers added WO3*H2O nanosheets to a well-dispersed graphene oxide solution and then stirred the solution to form a homogeneous suspension.
The WO3 was homogeneously embedded in the graphene interlayer, so the electrode had stable cycling performance because the volume expansion of WO3 could be effectively mitigated during the cycling process. The reversible capacity was maintained at around 615 mA h g-1 after 1000 cycles at a current density of 1080 mA g-1. Kim et al. reported a 2D nanocomposite composed of graphene nanosheets well scattered with WO3 nanosheets on top. After 50 cycles at 80 mA g-1, its capacity was 688.8 mA h g-1 compared to 555.2 mA h g-1 for pure WO3.
Gu et al. fabricated bamboo-like WO3 nanorods anchored on an N-doped three-dimensional graphene framework. This composite can effectively withstand volume changes and it provides higher electrical conductivity with excellent high-rate capability. Reduced graphene oxide (rGO), usually obtained by reducing graphene oxide, is widely used to achieve better electrochemical properties of tungsten oxides.
Dang et al. successfully embedded the WO3 nanosheets into the rGO matrix by hydrothermal method and heating treatment. After 150 cycles at 100 mA g-1, the discharge capacity was still 1005.7 mA h g-1, which is almost twice that of pure WO3 (565 mA h g-1). The main reason for this improvement can be attributed to the fact that rGO not only provides a more convenient channel for ions and electrons but also largely buffers the damage to its structure during cycling.
Huang et al. produced h-WO3 nanorods embedded in the rGO matrix doped with N and S. The composite possessed a specific discharge capacity of 1030.3 mA h g-1 in the first cycle and slightly decreased to 816 mA h g-1 in the second cycle at a current density of 100 mA g-1.
In addition, the specific discharge capacity averaged 196.1 mA.h.g-1 over 200 cycles at a high current density of 1500 mA.h.g-1. Park et al. dispersed WO3 particles on a three-dimensional macroporous rGO framework. This special structure and the good conductivity of rGO together improved its rate capability and cycling stability.
The specific discharge capacity was 440 mA.h.g-1 after 100 cycles at a current density of 100 mA.g-1. Kim et al. also realized a nanocomposite in which WOx nanoparticles were uniformly embedded in a mesoporous carbon matrix. Its main improvement is the low polarization during the desulfurization process due to the high electrical conductivity of the mesoporous carbon matrix and the shorter lithium diffusion pathway.
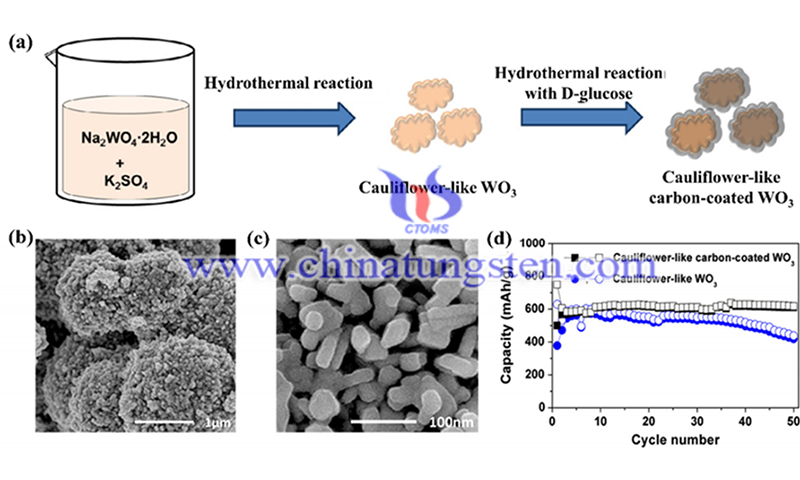
In addition to the tungsten oxide-carbon composites mentioned above, amorphous carbon materials are often used to achieve better electrochemical performance. For example, Yoon et al. coated a thin layer of carbon on the flower-like WO3, which enhanced the electrochemical correlation between the active WO3 and the current collector and also buffered the volume change, showing better cycling stability and rate performance than pure WO3.
Herdt et al. encapsulated an array of WO3 nanorods in a thin layer of carbon. The vertical alignment of the nanorods was maintained after 200 charge/discharge cycles at C/20, indicating that this composite has excellent structural stability. In addition, Liu et al. obtained a WO3*0.33H2O@C composite, in which WO3*0.33H2O was surrounded by amorphous carbon coating.
In their studies, the right amount of carbon coating can produce positive effects, while when the amount of carbon is too much, it is too much and reduces the crystallinity of WO3-0.33H2O as well as the capacity of the composite. In addition, it has been reported that ultrathin WO3-x nanoplates doped with carbon also exhibit excellent electrochemical properties.
Reference: Han W, Shi Q, Hu R. Advances in electrochemical energy devices constructed with tungsten oxide-based nanomaterials[J]. Nanomaterials, 2021, 11(3): 692.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com