Study Illustrates How Salt Simplifies Formation of 2D Molybdenum Disulfide
- Details
- Category: Tungsten's News
- Published on Tuesday, 26 April 2022 13:56
Salt (NaCl) acts an intermediary role in the chemical vapor deposition growth of 2D molybdenum disulfide (MoS2), accelerating its generation process. Materials theorists at Rice University found that the NaCl and precursors form eutectics with melting temperatures lower than either of them.
Boris Yakobson, a materials theorist from the Rice University lab, shows why in his 2018 follow-up study, which illustrates how NaCl simplifies first-principles analysis of the creation process of useful two-dimensional MoS2 and could enable more improvements to it.
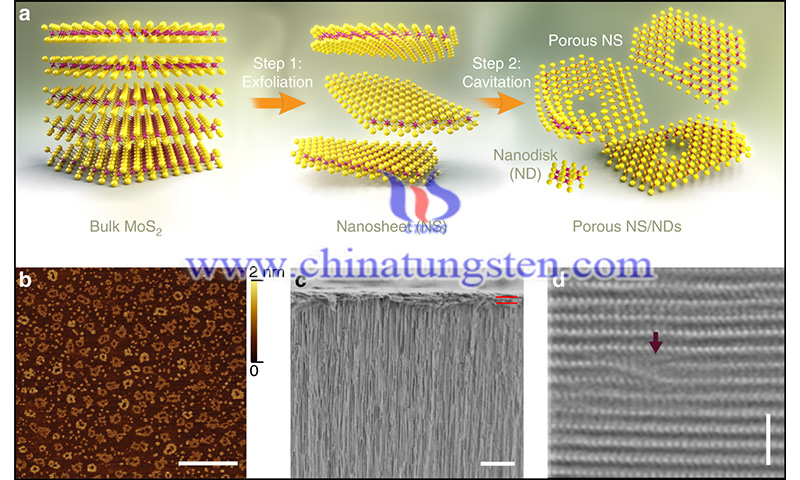
(Image Credit: Bedanga Sapkota/ Nature Communications)
Theoretical studies conducted by Yakobson and co-workers Jincheng Lei, Yu Xie, and Alex Kutana showed why salts, particularly iodized salts, tend to lower the reaction temperature in chemical vapor deposition (CVD) furnaces, which are considered critical for the development of MoS2, by simulating atomic-level energies.
Salt is used to provide more MoS6 by helping to skip several steps in conventional CVD growth and by skipping high-energy barriers, which is considered a necessary precursor for 2D MoS2. Their study, published in the Journal of the American Chemical Society, focused on how NaCl can reduce activation barriers to improve sulfation of molybdenum oxyhalides, the gaseous feedstock for MoS2 crystallization.
MoS2 is a natural compound known by the name pyromorphite. In addition, the two-dimensional form of MoS2 is favored for its semiconducting properties, which holds promise for the development of optoelectronics, electronics, spintronics, catalysis, and medical applications. However, two-dimensional MoS2 remains difficult to manufacture in commercial quantities.
Initially, Rice's group conducted research when laboratories in Singapore, China, Japan, and Taiwan used NaCl to create a so-called "library" of two-dimensional materials integrating transition metals and brass.
Good functionality of materials - even theoretical ones - is a challenge, prompting them to turn to the Yakobson lab's expertise in materials modeling. Their extensive modeling showed that while international labs use chlorine salts to create their materials libraries, iodide salts in general are more advantageous in accelerating the synthesis of MoS2.
The rapid and large-scale synthesis is essential for the widespread use of 2D molybdenum disulfide. We carefully studied the whole growth process in the hope of optimizing it as much as possible. It turns out that MoS2 can be synthesized faster at lower growth temperatures by simply changing the chloride to iodide.
This occurs when the salt and precursor combine to form a eutectic, a mixture of substances that melts and solidifies at a single temperature, which is lower compared to the melting point of the components.
After salt-assisted synthesis was shown to enable the growth of many TMD (transition metal dichloride) compounds more than previously possible and to significantly improve the growth conditions of previously synthesized compounds, it became clear that there was something special about this process.
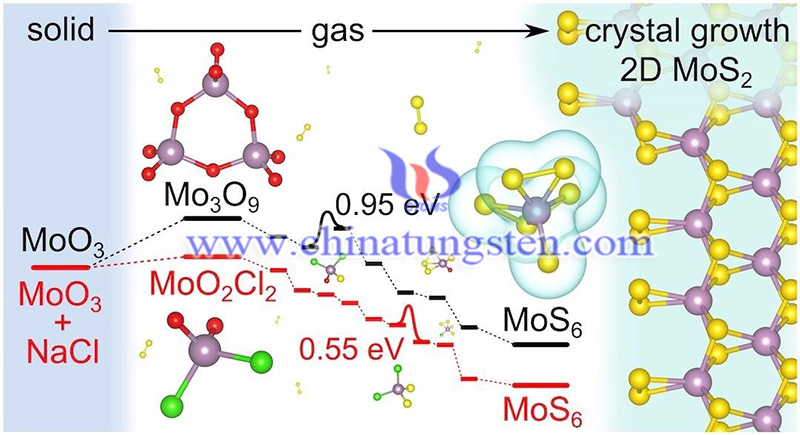
(Image Credit: Jincheng Lei/Yakobson Research Group)
Bets of Rice University's Department of Materials Science and Nanoengineering, adds, "Some experimental groups have tried to investigate further, but monitoring the molecular composition of the gas phase under growth conditions is not a simple task. Even then, you can't see the full picture."
"We were very thorough and followed up on Jincheng's work on the conventional 2D molybdenum disulfide growth mechanism. We simulated all parts of the process, from sulfation to two-dimensional crystal growth. This more comprehensive approach paid off," Bets added.
In the simulations, the group from Rice University was able to directly observe the complete sulfidation process as the chlorine and oxygen atoms in the general precursor MoO2Cl2 were gradually replaced by sulfur under CVD conditions. The lab says that eutectic effects may be considered a common phenomenon in the synthesis of two-dimensional dichloride monolayers by CVD, and thus worthy of continued study.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com