Rare Earth Nano Fluorescent Probe Detects COVID-19 Antibodies in 10 Mins
- Details
- Category: Tungsten's News
- Published on Monday, 26 October 2020 12:12
The first domestic rare earth nano fluorescent probe for COVID-19 detection has been developed and produced by Xiamen Aode Biotechnology Co., Ltd., and the probe has recently obtained the third-class medical device registration certificate issued by the State Drug Administration.
It is understood that Xiamen Aode Biotechnology Co., Ltd., located in Haicang District, is a rare earth biomedical enterprise incubated by the Xiamen Rare Earth Materials Research Institute of the Haixi Innovation Research Institute of the Chinese Academy of Sciences. It has a technical team led by Academician Hong Maochun and relies on the scientific research platform of the Chinese Academy of Sciences.
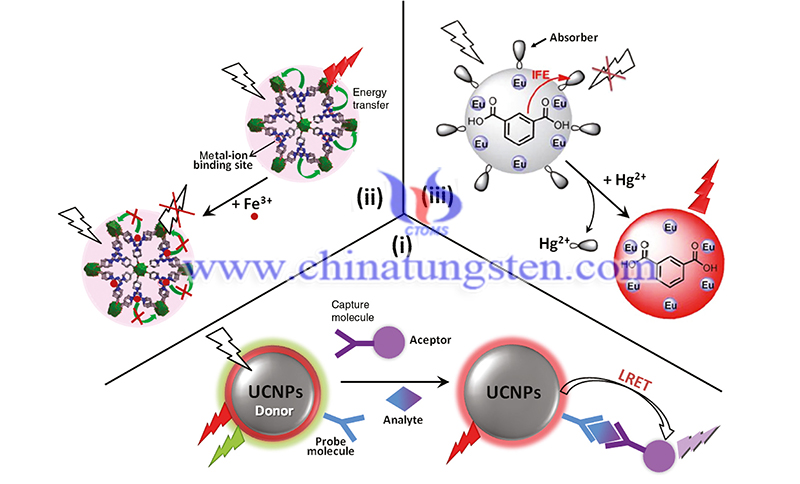
Biomedicine and cell and molecular biology require powerful imaging techniques of the single molecule scale to the whole organism, either for fundamental science or diagnosis. One of the most active fields of research in the past decade has thus been the development of rare-earth based nanoparticles, whose optical properties and low cytotoxicity are promising for biological applications.
Attractive properties of rare-earth based nanoparticles include high photostability, absence of blinking, extremely narrow emission lines, large Stokes shifts, long lifetimes that can be exploited for retarded detection schemes, and facile functionalization strategies.
The use of specific ions in their compositions can be moreover exploited for oxidant detection or for implementing potent contrast agents for magnetic resonance imaging. In this review, we present these different applications of rare-earth nanoparticles for biomolecule detection and imaging in vitro, in living cells or small animals. We highlight how chemical composition tuning and surface functionalization lead to specific properties, which can be used for different imaging modalities.
The company's 2019-nCoV IgM/IgG antibody determination kit (rare earth nano-fluorescence immunochromatography) developed based on the "rare-earth nanoparticle fluorescent probe" could achieve ultra-sensitive detection of new coronavirus antibody titer. The entire detection process can be completed in 10 minutes.
Under the guidance of the market supervision department, on September 29, the product formally obtained the third-class medical device registration certificate issued by the National Medical Products Administration (National Medical Products Administration 20203400776), becoming the first rare-earth nano-fluorescence approved by the National Medical Products Administration Probe COVID-19 detection reagent products.
It is reported that Xiamen Aode Biotechnology Co., Ltd. currently has some authorized patents in the field of rare earth biomedicine, and has successfully developed a series of rare-earth nano-fluorescence immunological in vitro diagnostic products with independent intellectual property rights. Among them, 27 products have passed CE certification, 32 rare-earth diagnostic kits and 2 rare-earth nano-fluorescence immunoassay analyzers have obtained the national medical device registration certificate.
The COVID-19 IgM/IgG antibody assay kit (rare earth nano-fluorescence immunochromatography, colloidal gold method) developed by Xiamen Aode Biotechnology Co., Ltd. has obtained CE, Russia GOST, Brazil ANVISA, and certifications from other countries, and has been supplied to many overseas countries successively.
- Rare Earth Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com