WS2 Composites
- Details
- Category: Tungsten Information
- Published on Monday, 29 August 2022 21:48
To improve the electrical and catalytic properties of WS2, the synthesis of WS2 composites from other materials with good electrical conductivity is a promising approach. Composites are materials in which one material is the matrix and another material is used as the reinforcement. The various materials complement each other in terms of properties and create a synergistic effect, resulting in an overall performance superior to the original material.
In recent studies, the most attention has been paid to the incorporation of nano WS2 into conductive carbon matrix by various synthetic methods, including graphene, carbon nanotubes, and amorphous carbon. In terms of synthesis, the hydrothermal method is the most commonly used method because of its simple steps.
Two-dimensional carbon materials are found to have high electrical conductivity and good flexibility. Therefore, the construction of homogeneous mixtures of highly conductive 2D carbonaceous nanosheets and WS2 nanostructures is expected to promote electron transport and improve the electrical conductivity of the WS2 composites.
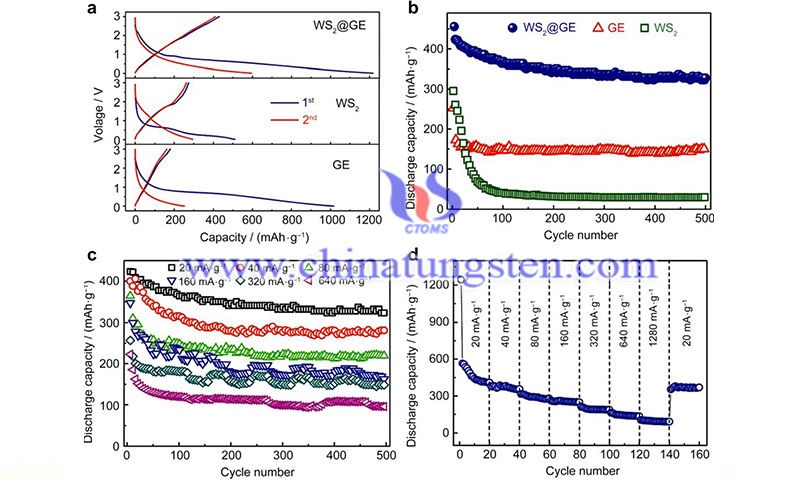
For example, Yang et al. synthesized WS2/reduced graphene oxide (RGO) hybrid nanosheets through a hydrothermal reaction process achieved at low temperatures, which demonstrated the connection with RGO support. Since graphene is a soft material with a low bending stiffness, it can be easily tuned into different morphologies.
In addition, studies have also reported the synthesis of WS2 nanotube/graphene hybrids (Gs) from graphene oxide (GO) solutions. In the subsequent hydrothermal reaction, the conversion from GO to Gs was performed, and the Gs could curl and cross-link to form a three-dimensional (3D) network. Similarly, Chen et al. reported a convenient and green synthesis method of WS2/RGO/WO3 hybrids by aerosol treatment. The crinkled morphology of RGO provides abundant defects and folds, which is considered one of the potential factors to improve the electrocatalytic performance of WS2.
In addition, CVD methods for the preparation of WS2 composites as well as for thickness control are also a better approach. Besides, carbon nanotubes (CNTs) are valuable candidates for bonding with WS2 because of their excellent mechanical strength, large specific surface area (50-450 m2.g-1), and good electrical conductivity compared to other conventional templates. Several approaches can be carried out to fabricate composite structures.
For example, Yue et al. synthesized multi-walled carbon nanotubes (MWCNTs-WS2) by a low-temperature hydrothermal reduction strategy. Lin et al. demonstrated a unique method to synthesize WS2 nanoparticles on vertically aligned CNTs by thermally annealing W-coated CNTs in sulfur vapor. Single-walled carbon nanotubes (SWCNTs) show mechanical flexibility and superior electrical conductivity, which are more favorable for charge transport than MWCNTs.
To date, several studies have been conducted on the construction of WS2-SWCNT composite structures. For example, researchers have synthesized three-dimensional WS2@SWCNT foams using a simple hydrothermal method and a subsequent freeze-drying process. Due to the elastic three-dimensional conductive network, it is possible to provide conductive pathways to facilitate the transfer of WS2 charges.
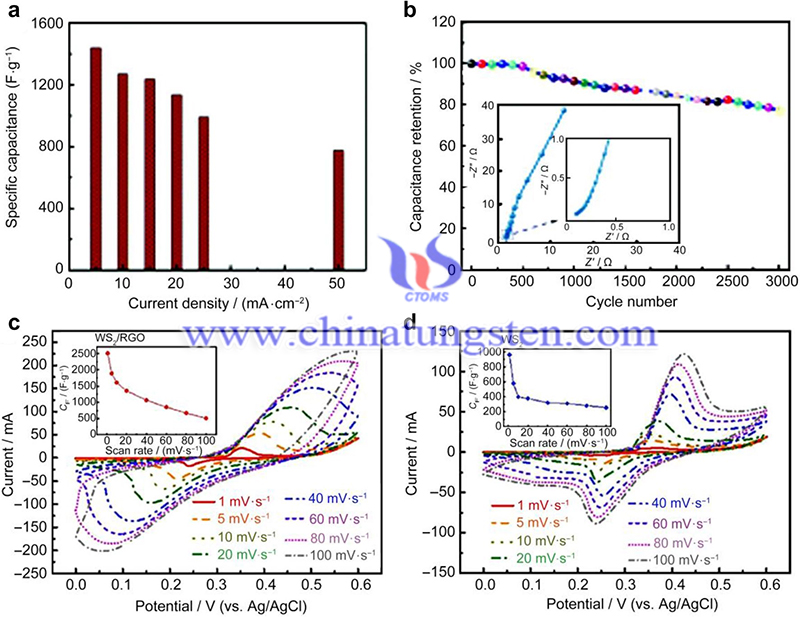
In addition, an innovative ternary WS2/CuO/SWCNT porous hybrid was fabricated by electrostatic-assisted self-assembly and vacuum filtration processes. In this hybrid, the positively charged CuO nanosheets are easily assembled with the negatively charged WS2 nanosheets and SWCNTs to form a flocculent network.
In addition, amorphous carbon has attracted sufficient attention due to its low cost compared to graphene and carbon nanotubes. Among them, commercially available Super P is a typical binding material to improve the electrochemical performance of WS2. As a conductive additive, the incorporation of Super P can help reduce the size and improve the dispersion of nanocomposites, which can accelerate the insertion/extraction reaction between WS2-Super P nanocomposite electrodes and electrolytes.
For example, Huang et al. prepared WS2-super P nanocomposite powders by a simple two-step process including hydrothermal and sulfide reduction reactions. In addition, Li et al. synthesized WS2 nanosheet-carbon (WS2/C) composites by a simple two-step process of ball milling and sulfide reaction.
Article Source: Sun, CB., Zhong, YW., Fu, WJ. et al. Tungsten disulfide nanomaterials for energy conversion and storage. Tungsten 2, 109–133 (2020).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com