Electrical Characteristic and HER Mechanism of Tungsten Disulfide Nanomaterials
- Details
- Category: Tungsten Information
- Published on Tuesday, 23 August 2022 20:46
Due to the promising applications of tungsten disulfide nanomaterials in the field of energy conversion and storage, efforts have been made to study and improve its electrical characteristic and HER mechanism of WS2, such as carrier concentration (p), mobility (μ), and resistivity (ρ). According to theoretical predictions, WS2 has the highest electron mobility in semiconductor TMDCs due to the reduced effective mass.
The electrical transport parameters of bulk 2H-WS2 measured at room temperature were found to be: ρ = 3.37 Ω-cm; p = 7 × 1016 cm-3; μ = 30 cm2 -V-1-s-1. The electron mobility can be improved when the thickness of WS2 is reduced to a single layer. For example, the electron mobility of multilayer and monolayer WS2 is 44 cm2 -V-1.s-1 at room temperature.
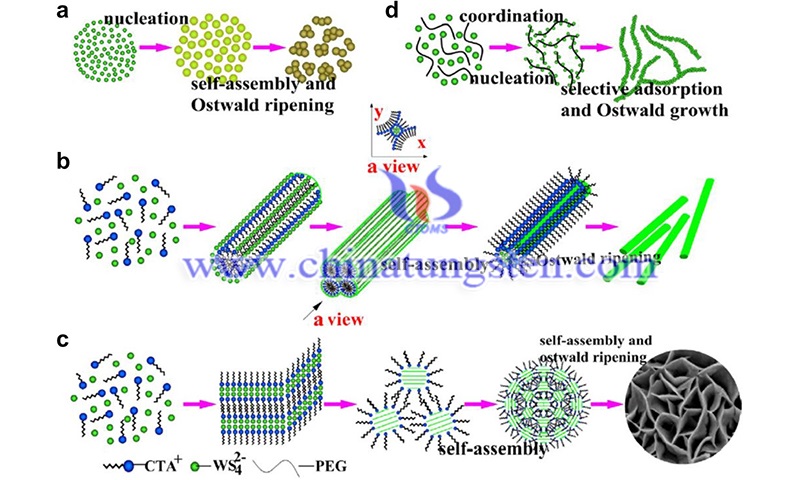
Similarly, the researchers studied the electronic properties of monolayer and bilayer WS2, obtaining field-effect mobility of 50 ± 7 cm2 -V-1.s-1 at room temperature. It should be noted that water molecules and other atoms absorbed on the surface of WS2 can act as traps for charge carriers, which can strongly affect the electrical properties of WS2. Therefore, in situ annealing can be used to remove adsorbed materials in order to study the intrinsic electrical characteristic of WS2.
In the case of WS2 nanotubes (NTs), the carrier mobility of NTs is six orders of magnitude smaller than that of bulk WS2 due to the non-ideal field-effect behavior. the carrier concentration of NTs varies from 3.0 × 1017 to 1.6 × 1018 cm-3, which is higher than that of bulk WS2 (1 × 1017 cm-3). The possible reason for the high carrier concentration of WS2 NTs compared to the bulk may be due to the strain and defects in the curved layer.
HER mechanism, the cathodic half-reaction of water decomposition, is one of the most intensively studied electrochemical reactions. To drive the reaction, additional energy is required to overcome the activation barrier, such as electrical energy. In the presence of an applied current, the electrocatalytic HER process of WS2 proceeds mainly through hydrogen adsorption (Volmer reaction) and desorption (Heyrovsky or Tafel reaction).
The ideal catalyst has a free energy of adsorbed atomic hydrogen close to thermal neutrality (GH≈0), which may affect the first step of the HER mechanism process. Based on DFT calculations, the researchers calculated the hydrogen binding energy of WS2, where the S and W sides are equal (GH ≈ 0.22 eV), which indicates that WS2 should be a reasonably good hydrogen evolution catalyst.
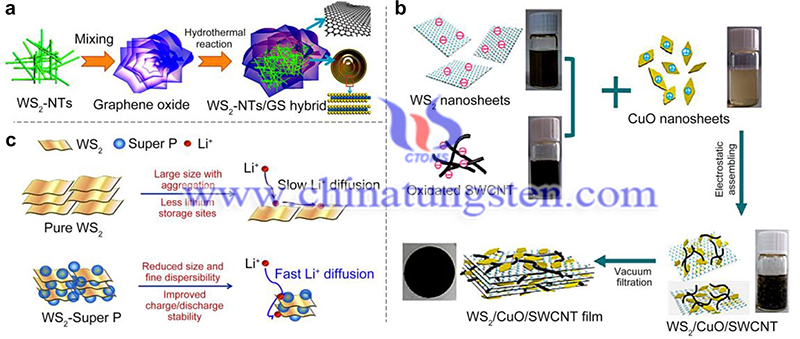
The edge of WS2 is the active site and the crystal structure has a considerable influence on the activity of HER, especially the 1T structure. For example, Voiry et al. found that the metal 1T site is an important factor in enhancing the catalytic activity of tungsten disulfide nanomaterials. This can be attributed to the fact that 1T-WS2 becomes highly distorted with a large percentage of zigzag strain in the lattice. the atomic hydrogen adsorption free energy of 1T-WS2 monolayers can be modulated by the strain.
Article Source: Sun, CB., Zhong, YW., Fu, WJ. et al. Tungsten disulfide-based nanomaterials for energy conversion and storage. Tungsten 2, 109–133 (2020).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com