Oxidation and Reduction of Tungsten and Its Oxides
- Details
- Category: Tungsten Information
- Published on Monday, 28 March 2022 19:15
Tungsten (W) is mainly in the W+6 oxidation state in most W oxides, with six oxygen atoms surrounding each W atom in an octahedral configuration. In oxidized tungsten (WO3), these octahedra are arranged in a split-angle configuration. In reduced oxides (WOj, 2 < x < 3), complex combinations of WO6 octahedra in split-angle, split-edge, and split-face arrangements are frequently found. The WO4 tetrahedra and WO7 pentagonal dihedra, which are frequently found in fully oxidized and partially reduced compounds, respectively, add to the complexity of the crystal geography of these compounds.
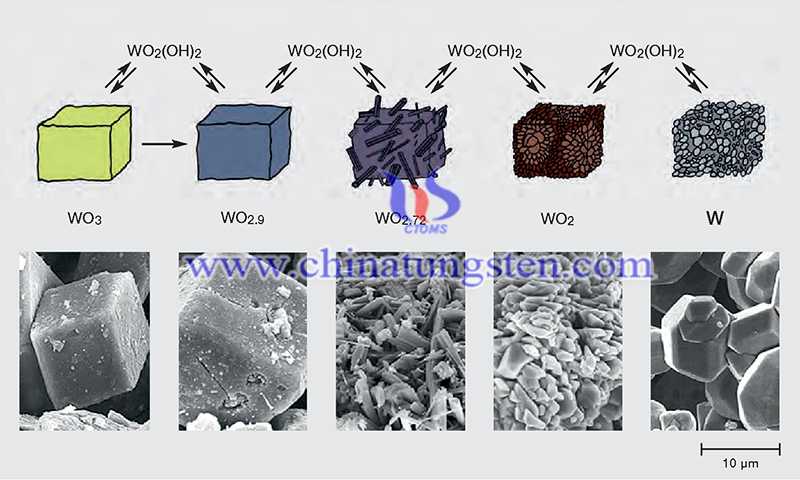
With different valence states, hundreds or thousands of W compounds appear. W is easily oxidized in air or oxygen to form compounds. This reaction forms WO3 when the temperature does not exceed 327°C. The thickness of the oxide layer depends on the temperature and humidity. From 327°C to 400°C, a protective oxide layer is formed, although there is some doubt about its composition (it is said to be WO2.75, but this number is also disputed).
Above 600 °C, a WO3 layer is produced as the initial protective oxide is completely oxidized. WO3 is highly permeable to oxygen, so the WO2.75 layer continues to be oxidized to the nitrous oxide form at a rate governed by the diffusion of W ions to the WO2.75-WO3 boundary.
Sublimation of WO3 begins at 750°C and reaches an oxidation rate above 1300°C, resulting in a tungsten surface free of oxides above this temperature. It has been shown that the logarithm of the oxidation rate from 700 to 1300°C is proportional to PJ2/T, where P02 is the partial pressure of oxygen and T is the absolute temperature.
The oxidation of W with water and the reduction of W compounds with hydrogen are very similar, with the high partial pressure of water or hydrogen driving the reaction in a specific direction.

This reaction with water at 38°C is very slow and increases with increasing temperature and pressure. The reaction with water vapor between 20 and 500°C leads strictly to the formation of WO3 - no other compounds are formed. The rate of this reaction has been found to depend on temperature and the partial pressure ratio of water to hydrogen. However, by properly adjusting these partial pressures, all known compounds can be formed.
When both are very low, WO2 is formed. as these pressures increase, more oxidized forms are produced (WO2.72, WO2.9, and finally WO3). In addition, higher temperatures favor more oxidized forms. It is also important to note that hydrated oxides volatilize readily above 900°C, with the most volatile form being WO2(OH)2.
The study titled “Synthesis of tungsten carbide from bimodal tungsten powder produced by electrical explosion of wire” has been published in the journal International Journal of Refractory Metals and Hard Materials 103 (2022). The study was carried out by A.V. Pervikov, M.G. Krinitcyn, E.A. Glazkova, N.G.Rodkevich, M.I. Lerner at the University of Bath.
By adding hydrogen to the system, the later reaction becomes favorable and the tungsten oxide will be reduced rather than oxidized. Due to the volatility described above, the reaction conditions can significantly change the morphology of the final product. For example, the conditions of the reaction can be adjusted to reduce WO3 to W.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com