Synthesis of Spherical Macroporous WO3 Particles
- Details
- Category: Tungsten Information
- Published on Friday, 17 September 2021 01:00
- Written by yuntao
- Hits: 1498
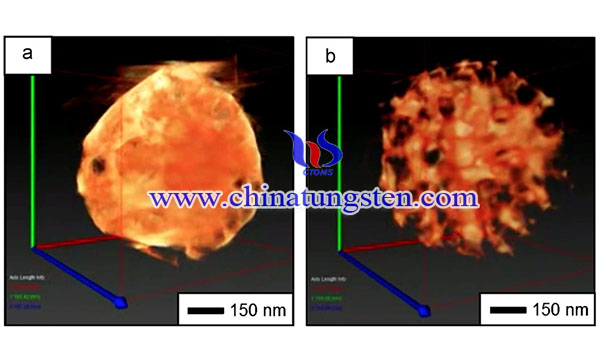
Tungsten trioxide (WO3) has been extensively studied due to its affinity for visible light, chemical inertness, thermal stability, and harmlessness. These excellent properties make this material useful for solar-related applications such as photocatalysts, solar cells, water splitting, and hydrogen generation.
Au-Modified Tungsten Trioxide and Its Gas Sensing to NOx
- Details
- Category: Tungsten Information
- Published on Friday, 17 September 2021 00:36
- Written by yuntao
- Hits: 1362
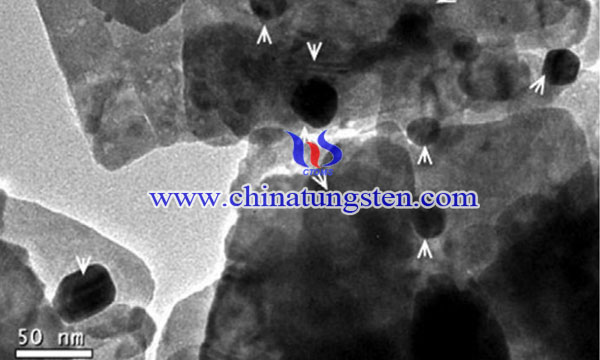
Semiconductor metal oxides (SMOs) are highly potent gas sensors for gaseous detection in terms of screening of air eminence, low expenditure on synthesis and sensing property that can be modified. The semiconductor metal oxide gas sensor is considered the most capable gas-sensing device due to its high sensitivity, fast response, low cost, and small size.
Read more: Au-Modified Tungsten Trioxide and Its Gas Sensing to NOx
WO3–Pt/C Electrocatalysts for Oxygen Reduction Reaction
- Details
- Category: Tungsten Information
- Published on Monday, 13 September 2021 14:21
- Written by yuntao
- Hits: 1720
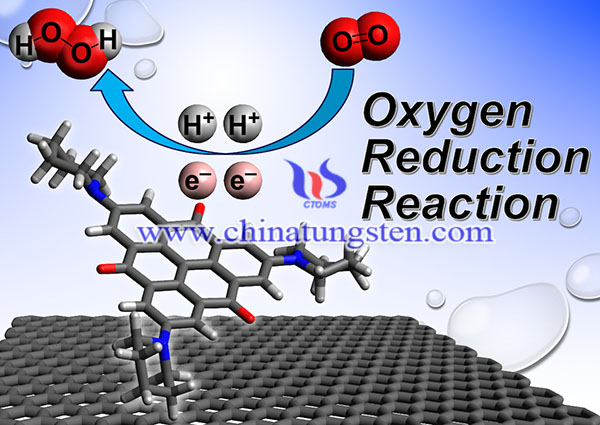
Oxygen reduction reaction (ORR) has been widely studied for its applications in fuel cells. There is a growing demand for clean energy technologies such as fuel cells. Since the energy efficiency and battery voltage of electrochemical cells are limited by the slow kinetics of ORR.Currently, platinum dispersed on carbon (Pt/C) is the most active catalyst for ORR; however, in addition to its high cost, this metal also has problems with ORR overpotential and poor methanol tolerance. Supporting by transition metal oxides such as tungsten trioxide (WO3) and titanium dioxide (TiO2) to the Pt/C electrocatalyst provide not only active sites but also electronic and ionic conductivity.
Read more: WO3–Pt/C Electrocatalysts for Oxygen Reduction Reaction
Growth of Tungsten Trioxide on Carbon Nanowalls
- Details
- Category: Tungsten Information
- Published on Friday, 17 September 2021 00:05
- Written by yuntao
- Hits: 1563

Tungsten Carbide Hollow Microsphere Prepared via Ammonium Metatungstate as Electrocatalyst Support
- Details
- Category: Tungsten Information
- Published on Monday, 13 September 2021 11:55
- Written by yuntao
- Hits: 1593

Hydrogen (H2) is widely concerned as the most potential candidate due to its high energy density and environmentally friendly reaction process. Electrochemical water splitting is a promising hydrogen production technology because it does not contain greenhouse gases and is economical and efficient. As a key semi-reaction process, the hydrogen evolution reaction (HER) plays a decisive role in the efficiency and cost of hydrogen production through electrochemical water splitting.





 sales@chinatungsten.com
sales@chinatungsten.com