Factors of Hydrothermal Method Preparing Hexagonal Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 10 August 2016 09:33
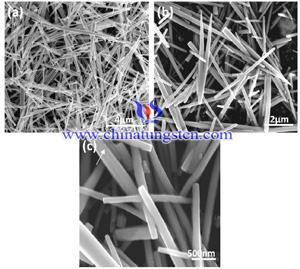 Hydrothermal process is carried in a special closed reaction vessel (reactor), the aqueous solution as medium, by heating the reaction vessel to create a high-temperature, high-pressure reaction environment to make the generally insoluble or poorly soluble material to dissolve and recrystallization, and then by filtering, washing, drying and other separation means to get the ultra-fine, high-purity particles. Hydrothermal method for preparing hexagonal tungsten trioxide, its crystalline form is affected by many factors, such as pH value, hydrothermal temperature and additives and others, the following will specifically analyze the impact of various factors.
Hydrothermal process is carried in a special closed reaction vessel (reactor), the aqueous solution as medium, by heating the reaction vessel to create a high-temperature, high-pressure reaction environment to make the generally insoluble or poorly soluble material to dissolve and recrystallization, and then by filtering, washing, drying and other separation means to get the ultra-fine, high-purity particles. Hydrothermal method for preparing hexagonal tungsten trioxide, its crystalline form is affected by many factors, such as pH value, hydrothermal temperature and additives and others, the following will specifically analyze the impact of various factors.
In the hydrothermal environment to prepare tungsten trioxide, the existence of hydrogen ions (H+) is one of the important factors which affect crystal form of product, especially when the reaction condition is acidic environment. The influence mechanism of pH on tungsten trioxide crystal form is really complex, it is generally believed that when in a different pH environment, the solution ion balance is changed, so that the crystal growth environment changes thus to achieve the purpose of crystal form control, however the specific impact mechanism is still unknown. Studies have shown that, hexagonal tungsten trioxide will be generate in the pH value range of among 1.5~2.0; In addition, tungstate ions will be partially polymerized in the acidic environment to generate paratungstate or metatungstate ions, which is not conducive to the generating of nanoribbons, experiment results also showed that, pH in the range of 11~12, the resulting product was hexagonal tungsten trioxide nanoribbons.
Study found out that when the hydrothermal temperature is among 200 ~ 300 ° C, hexagonal tungsten trioxide will be prepared by the hydrothermal method; and when the temperature reaches to 350°C, the resulting product will be orthorhombic tungsten trioxide. The polymorph regulation of hydrothermal temperature on the tungsten trioxide depends on the energy of system changing. Moreover, a study that taking sodium tungstate and HCl as raw materials, citric acid as an additive, the result shows that hexagonal WO3.2H2O will generate at hydrothermal temperature of 150°C, however, the 180°C will get hexagonal WO3.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com