Spectrophotometry Determine Fe Element in Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 17:06
 Spectrophotometry is qualitative and quantitative analysis method for the substance by measuring the absorbance or emission intensity of the test substance in a specific wavelength or range of wavelengths of light. When the solution is a bunch of intensity I0 monochromatic vertical irradiation of a substance, due to the portion of the light is absorbed by the system, thus the intensity of the transmitted light is reduced to I, T is the light transmittance of the solution is: according to Lambert (Lambert) - Beer (Beer) Law: A = abc. Where A is the absorbance, b is the thickness of the solution layer (cm), c is the concentration of the solution (g / dm ^ 3), a is the extinction coefficient. There are relationships between the absorption coefficient of the nature of the solution, temperature and wavelength and other factors. Solutions of other components (solvents, etc.) are available to deduct by blank liquid absorption of light ion.
Spectrophotometry is qualitative and quantitative analysis method for the substance by measuring the absorbance or emission intensity of the test substance in a specific wavelength or range of wavelengths of light. When the solution is a bunch of intensity I0 monochromatic vertical irradiation of a substance, due to the portion of the light is absorbed by the system, thus the intensity of the transmitted light is reduced to I, T is the light transmittance of the solution is: according to Lambert (Lambert) - Beer (Beer) Law: A = abc. Where A is the absorbance, b is the thickness of the solution layer (cm), c is the concentration of the solution (g / dm ^ 3), a is the extinction coefficient. There are relationships between the absorption coefficient of the nature of the solution, temperature and wavelength and other factors. Solutions of other components (solvents, etc.) are available to deduct by blank liquid absorption of light ion.
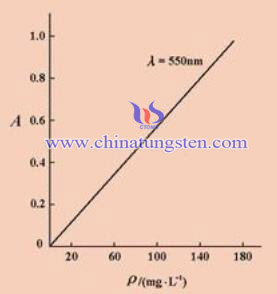
From the above equation, when the thickness of the solution layer b and a are fixed a, which can show a linear relationship in the concentration of the solution and the absorbance A. When we have quantitative analysis, determine a solution for absorption of different wavelengths of light (absorption spectrum) is needed, which determines the maximum absorption wavelength. We take this wavelength of light as the light source, a series of known concentrations measured absorbance c solution A, and making A ~ c curve. In the analysis of an unknown solution that is based on the measured absorbance A, checking the working curve we can determine the corresponding concentrations. This is the basic principle of spectrophotometry measurement of concentration.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
We find the stability enables the method sensitivity, contrast and enhanced complex improving in the test of 5-Br-PADAP-Pe (Ⅱ) system that adding non-ionic surfactant emulsifier OP and ethanol. In the conditions of 5-Br-PADAP-Fe (Ⅱ) complex formation emulsifier OP pluralism, and in the presence of ethanol and an emulsifier OP, pH3.5-10H Ac-NaAc buffer medium, Fe (Ⅱ) with 5-Br -PADAP generates purple complex at a wavelength of 558nm, the Fe content in the 0-60μg / 50ml law is obeyed in the range, the complex apparent molar absorption coefficient is 7.64 × 10 ~ 4. Color remains completely within five minutes, the absorbance is stable within 24 hours at room temperature, this method has good selectivity.



 sales@chinatungsten.com
sales@chinatungsten.com