Sodium Tungstate Dihydrate-XIII
- Details
- Category: Tungsten Information
- Published on Tuesday, 30 June 2015 17:17
f) Oxidation of organosulfur compounds
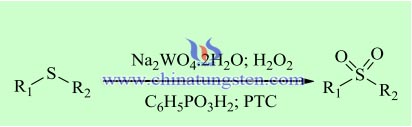
The oxidation of organosulfur compounds is the most straightforward method for the preparation of sulfoxides and sulfones, important as commodity chemicals or pharmaceuticals. Noyori and co-workers reported an oxidative process using sodium tungstate dihydrate as catalyst, 30% aqueous H2O2 as oxidant, and Q+HSO4- as PTC, besides phenylphosphonic acid as a promoter of the biphasic oxidation, which converts several aromatic and aliphatic organosulfur compounds into the corresponding sulfoxides and sulfones without organic solvents. With this method diphenyl sulfide is converted into diphenyl sulfone with 96% yield. The authors studied others substrates, the aliphatic sulfides being more reactive than the aromatic counterparts. Moreover, the substrates with allyl groups or with primary or secondary alcohols are oxidized to the corresponding sulfones, the allylic and the alcohol groups being unaffected.
Tungsten Powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn



 sales@chinatungsten.com
sales@chinatungsten.com