Most Common Form of Tungsten Disulfide Nanomaterials: Nanosheets
- Details
- Category: Tungsten Information
- Published on Thursday, 25 August 2022 16:35
Nanosheets of tungsten disulfide nanomaterials are the most common form, and the main synthetic strategies can be divided into two categories: top-down and bottom-up approaches. Top-down approaches allow the production of small amounts or single-layer samples at a lower cost, which is very beneficial for basic research. Among these top-down methods, mechanical peeling via Scotch tape is the simplest method, with only a few or single layers of WS2 exfoliated via Scotch tape.
However, large-scale production is extremely challenging and yields are very limited. In addition, WS2 can be exfoliated in the liquid phase, which requires the assistance of ultrasound or ion intercalation: ultrasonic treatment typically results in crystal flakes with large dimensions of several hundred nanometers. Therefore, a more feasible approach is to use ion intercalation into WS2 atomic layers to separate the flakes
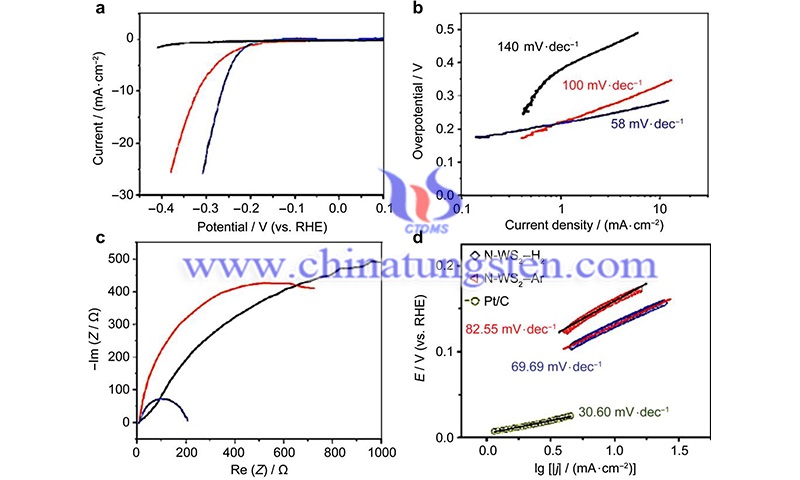
For the aforementioned yield and size limitations, several techniques of bottom-up strategies are reported for the controlled fabrication of nanosheets on a given substrate by staring from the constituent elements of WS2. Most of the proposed processes utilize precursors in vapor form and transport selected species on the substrate via a carrier gas.
Chemical vapor deposition (CVD) is a widely used technique for preparing WS2 nanosheets, which generally uses WO3 as the W source and S powder or H2S as the S source. For example, Hu et al. prepared WS2 on SiO2/Si substrates by a conventional low-pressure CVD method and proposed a corresponding growth mechanism.
In addition, atomic layer deposition (ALD) is a modified CVD technique in which two precursors are set up separately in the growth chamber, and its main advantage is the controllable thickness of WS2 by varying the number of cycles of ALD. ALD is still not widely used to fabricate WS2 nanosheets, mainly due to the lack of a suitable ALD chemistry.
Due to the instability of planar nano-S-W-S sheets of tungsten disulfide nanomaterials with a large number of overhanging bonds at the edges of the layers, closed structures such as IF-WS2 and NT-WS2 are formed by specific preparation methods. The preparation methods for producing IF-WS2 nanoparticles and NT-WS2 are broadly divided into two groups: (1) processes such as arc discharge, electron beam irradiation, and laser ablation, and (2) chemical methods close to equilibrium, such as chemical gas-solid reactions.
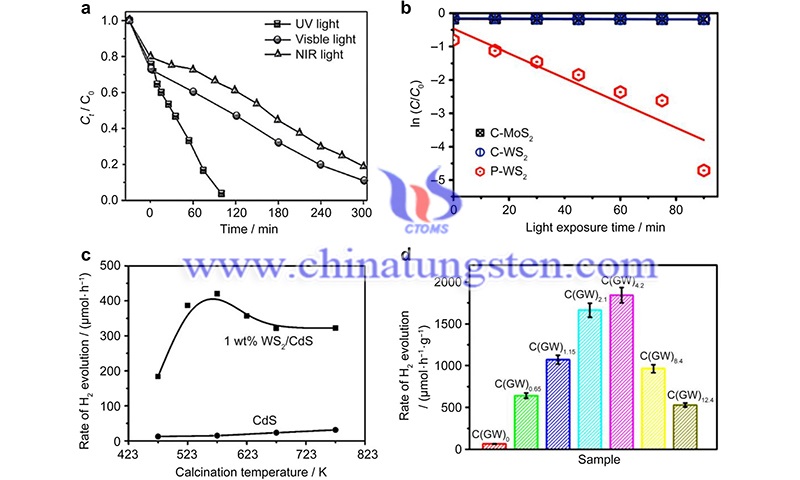
Article Source: Sun, CB., Zhong, YW., Fu, WJ. et al. Tungsten disulfide nanomaterials for energy conversion and storage. Tungsten 2, 109–133 (2020).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com