Molybdenum Doped Bilayer Photoanode Nanotubes Enhance Photoelectrochemical Water Splitting
- Details
- Category: Tungsten Information
- Published on Saturday, 02 April 2022 19:49
Researchers from the University of Pittsburgh recently enhanced the photoelectrochemical (PEC) water splitting capability of bilayer photoanode nanotubes through simple but effective molybdenum (Mo) doping method.
The study titled “Molybdenum doped bilayer photoanode nanotubes for enhanced photoelectrochemical water splitting” has been published in the international journal of hydrogen energy 47 (2022). The study was carried out by Shrinath Dattatray Ghadge, Moni K. Datta, Oleg I. Velikokhatnyi, Prashant N. Kumta.
Continued global population growth and rapidly decreasing fossil fuel reserves, coupled with health and environmental concerns caused by worrisome air pollution, make the exploration of alternative, renewable, and environmentally friendly energy resources urgent. In this context, hydrogen has been hailed as one of the most promising energy sources to replace traditional fossil fuels due to its non-carbon nature and higher energy density than fossil energy sources.
Currently, on the one hand, large-scale hydrogen is produced by steam reforming of natural gas, partial oxidation of hydrocarbons, and coal gasification. However, these conventional methods of hydrogen production suffer from serious drawbacks. In addition, the higher operating temperatures of these methods increase the production costs. The hydrogen produced by these methods is not pure and is often contaminated with pollutants. On the other hand, electrolysis from water is currently a highly reliable method for the production of clean and non-hydrocarbons.
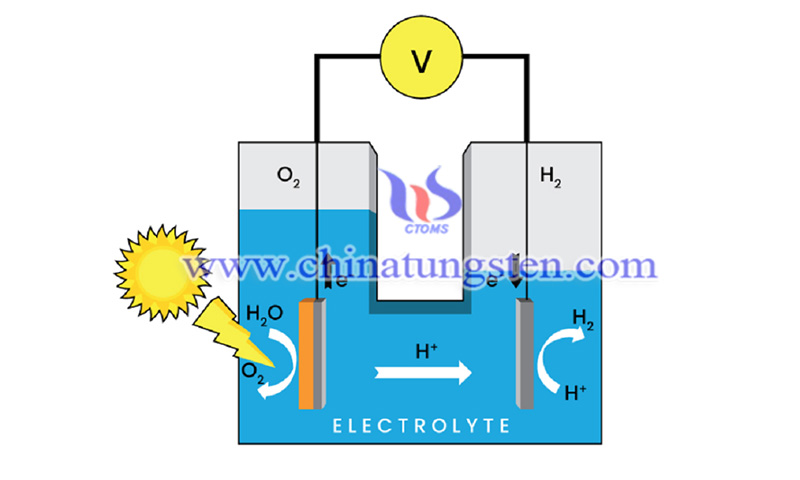
Hydrogen production from water splitting using solar-based photoelectrochemical is one of the most reliable options to address the growing energy and global warming crisis. Since water splitting is an endothermic reaction, it requires external energy to split water into hydrogen and oxygen. Since the discovery of PEC water splitting by low-cost semiconductor materials, namely titanium dioxide (TiO2), by Fujishima and Honda in 1972, much attention has been paid to the design of new high-performance semiconductor photocatalysts to improve the overall performance of PEC cells.
As a result, various semiconductor systems have emerged, such as single metal oxides (WO3), bimetallic oxides, brassides, and nitrides. Two-dimensional materials (graphene, MoS2) and carbon-based nanomaterial catalysts have been investigated for PEC water splitting. Among these different photoactive systems, transition metal oxides (TMO) are very promising for applications in PEC water splitting due to their high chemical stability, good electron mobility, natural abundance, and low cost.
However, TMO-based systems often encounter several problems that limit the high performance of PEC cells. Among the various TMOs, the n-type semiconductor WO3 is a versatile material with good thermal stability, photosensitivity, stability against photo-corrosion, and good electron transport properties. Further enhancement of PEC activity can be achieved by many strategies, such as morphology control, transition metal doping, and surface sensitization. Among these different approaches, doping is an effective strategy to tune the optical, structural, and electronic properties of WO3.
Among the different dopants, Mo is a well-studied and very promising candidate dopant for WO3. Therefore, in this work, the team chose Mo-doped WO3 as a highly efficient bilayer photoanode system for further study.

The molybdenum-doped ones have a higher light absorption capacity compared to the undoped bilayers. In addition, the addition of Mo improved the charge carrier density and photocurrent density and reduced the charge transfer resistance. In addition, the doped bilayer photoanodes showed good long-term PEC stability under light, indicating their robustness in PEC water separation.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com