WO3/ZnO Nanorods for Photocatalytic Degradation of Herbicides: Ammonium Paratungstate as Raw Material
- Details
- Category: Tungsten Information
- Published on Saturday, 21 August 2021 00:45
The chlorinated phenoxyacetic acid herbicide 2,4-D is extensively used for control of broadleaf weeds in cereal crops. It has been used for weed control on wheat, barley, oats, rice, maize and raps crops and is typically applied to cereals at a rate (active ingredient) of 0.5 kg ha−1. 2,4-D is potentially dangerous to both animals and humans and is a well-known endocrine disruptor.
Photocatalytic conversion of solar energy and remediation of toxic pollutants by employing photocatalysts have gained a lot interest. Zinc oxide (ZnO) is a excellent semiconducting material due to its high photocatalytic capability, environmental stability and low cost. However, it exhibits large band gap (∼3.3 eV) which make it only functions under irradiation with ultraviolet (UV) light. Many efforts have been made to extend the optical response of ZnO into visible light region by doping with transition metal elements to the Zn sites, or N, C and S to the oxygen sites, or coupling of ZnO with other narrow band gap semiconductors.

Tungsten trioxide (WO3) is one of the most attractive candidates for photocatalysis, as its bandgap ranges between 2.4 eV and 2.8 eV. Notably, the valence band edge of WO3 is about +3.1 VNHE, which could drive the oxidation of water or hydroxide ions to radical radical dotOH. Hence, WO3 is particularly suitable for the degradation of organic contaminant. In addition, WO3 possesses the advantages of low cost, nontoxicity, and high chemical stability, which does not lead to secondary pollution.
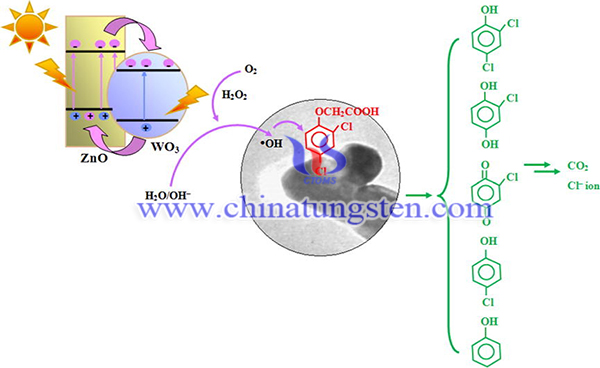
In order to enhance the photocatalytic performance, WO3 has been coupled with ZnO nanorods (NRs) by a hydrothermal-deposition method. WO3/ZnO nanorods are fabricated for photocatalytic degradation of herbicides using ammonium paratungstate as tungsten source, the as-prepared catalyst has excellent photocatalytic degradation of 2,4-dichlorophenoxyacetic acid (2,4-D). The preparation method of WO3/ZnO photocatalyst is as below:
Firstly, 1.0 g of pure ZnO NRs was dispersed in 50 mL deionized water and the suspensions were ultrasonicated for 30 min. The pH of the mixture was adjusted to about 6.5 using ammonia solution. After that, ammonium metatungstate with different W/Zn molar ratios (0.5%, 1.0%, 2.0% and 5.0%) was added into above mixture, followed by stirring for 12 h. Subsequently, the as-formed precipitates were filtrated, washed with deionized water and ethanol for several times, dried in air at 60 °C for 12 h and finally calcined at different temperatures (300 °C, 400 °C and 500 °C) for 2 h. As a comparison, the pure ZnO NRs were also calcined at 300 °C for 2 h without the addition of ammonium metatungstate.
In summary, WO3/ZnO nanorods are fabricated for photocatalytic degradation of herbicides using ammonium paratungstate as tungsten source, the as-prepared catalyst has excellent photocatalytic degradation of 2,4-dichlorophenoxyacetic acid (2,4-D). The WO3 loading and calcination temperature exerted great effect on photocatalytic activity. The sample contained 2.0% WO3 and calcined at 300 °C displayed the highest photocatalytic activity with a degradation rate of 0.291 min−1, which was 1.8, 4.3 and 17.7 times higher than those of pure ZnO NRs, commercial TiO2 and commercial WO3, respectively.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com