WO3/TiO2 Photocatalytic Material with Enhanced Photocatalytic Properties Prepared via APT for Degradation of Rhodamine B
- Details
- Category: Tungsten Information
- Published on Wednesday, 14 July 2021 04:29
As the demands for solar energy, pure air and water, and disposing of poisonous and hazardous pollutant are increasing, the use of semiconductor photocatalysts for environmental purification has attracted considerable attention due to their applicability for the removal of a wide variety of pollutants. Especially TiO2 has been studied widely in virtue of the high photocatalytic activity for organic pollutants such as RhB, tetracycline, phenol, and methylene blue under UV irradiation. Although TiO2 has various merits, the practical application of TiO2 is limited by its low photon utilization efficiency and the need for an ultraviolet excitation source which accounts for only a small fraction of solar light (approximately 5%).
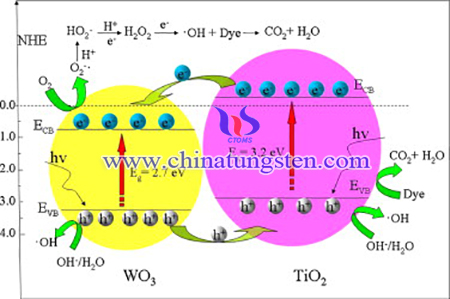
One of the common methods to enhance the properties of TiO2 is by doping with semiconductor metals. Tungsten trioxide (WO3) is a promising doping material due to its suitable band gap (Eg ≈ 2.7 eV) for visible light absorption, and the high oxidation power of photogenerated holes in valence band. In order to improve the photocatalytic properties of TiO2, WO3/TiO2 photocatalytic material with enhanced photocatalytic properties has been prepared via ammonium paratungstate (APT) for degradation of Rhodamine B.
The preparation method of WO3/TiO2 photocatalytic material is as following steps: A solution containing 80 ml of ethanol and 10 ml of iso-propanol was mixed thoroughly with 2 ml of titanium (IV) butoxide. The mixture was slowly dropped into 50 ml of deionised water to form a white precipitate. The reaction mixture was kept in a water bath with constant stirring at 90 °C and 10 ml of APT solution was added and thoroughly mixed. The excess water was evaporated with continuous stirring. The resultant precursor was then dried at 110 °C and finally calcined at different temperatures for 2 h in an air atmosphere. The sample was named as 2 wt% WO3/TiO2. Similarly, 5, 10 and 20 wt% WO3/TiO2 were also prepared by repeating the above procedure.
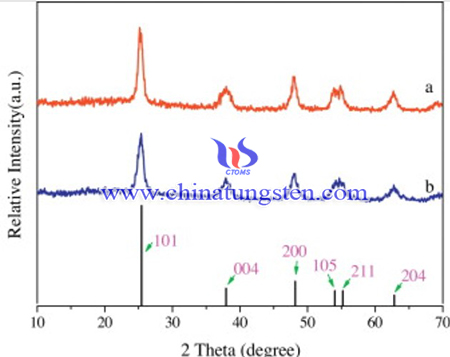
X-ray diffraction (XRD) measurements were carried out to investigate the crystallographic properties on a Rigaku D/MAX-2500 X-ray diffractometer with copper Kα radiation (λ = 1.54178 Å). Diffraction patterns were recorded in the angular range of 10–70°. Surface elemental analysis of the samples was carried out using a JEOL-JEM 2300 (LA) scanning electron microscope with an electron dispersion spectroscope (SEM-EDS) attachment. Adsorption–desorption isotherms of N2 on solid samples were measured at 77 K on a Micromeritics ASAP2020 apparatus, from which the Brunauer–Emmett–Teller (BET) surface area was calculated in terms of the multipoint BET method. X-ray photoelectron spectroscopy (XPS) surface characterizations were performed on a VG ESCALAB-MK electron spectrometer with Al Kα source. The subtraction of the energy shift due to electrostatic charging was determined using the contamination carbon C 1s band at 284.6 eV as a reference. Photoluminescence (PL) spectra were recorded on a RF-5301 florescence spectrophotometer (Shimadzu). The UV–vis absorption spectra of the samples were recorded on Perkin Elmer UV/vis spectrophotometer.
In summary, WO3/TiO2 photocatalytic material with enhanced photocatalytic properties has been prepared via APT for degradation of Rhodamine B. The degradation rate could reach 0.0201 min−1. The structural properties of composites have been altered obviously because of the tungsten oxide incorporation. By the photocatalytic experiment, the results show that 5 wt% WO3/TiO2 composites show superior photocatalytic degradation of RhB under visible light, and by comparison.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com