Single-Walled Carbon Nanotubes Fabricated By Decomposing Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Thursday, 08 July 2021 08:35
Single-walled carbon nanotubes (SWCNTs) have been an area of intense research since their discovery in 1993, owing to their extraordinary mechanical and unique electronic properties. It has been applied in fields including field-emission, SPM tips, and sensors. Catalyst is the decisive factor in fabrication of SWCNT by catalytic chemical vapor deposition (CCVD). The yield, purity, and textural properties of as-prepared SWCNTs were largely relied on the composition of the catalysts, the type of support material used, and the nature of the metal in the catalysts.
Thus, a production method of Single-walled carbon nanotubes (SWCNTs) with excellent properties were fabricated from W–Co–MgO catalysts by decomposing ammonium paratungstate (APT), magnesium nitrate, citric acid, and cobalt nitrate. And the result proved that the W1–Co5 catalyst is infectious for the synthesis of SWCNTs.
The preparation methods are as follows: A mixture of magnesium nitrate (Mg(NO3)2⋅6H2O), ammonium paratungstate ((NH4)5W12O38⋅5H2O), citric acid (C6H8O7⋅H2O), and cobalt nitrate (Co(NO3)3⋅9H2O) were dissolved in deionized water. In our experiment, the weight ratio of 15:0.15m (m=1,2,3):6:0.25n (n=3,5,10,15):1.5 (the molar ratio of W:Co:MgO=m:n:100) was used to fabricate the W–Co–MgO catalysts (termed as , respectively). After mixing at around 90 ∘C for 4 h, the catalyst was baked at 150 ∘C for 12 h, and then ground in a mortar to break the chunks into powder. The powder was placed into a quartz tube, and then heated up to 550 ∘C, and then air was introduced into the quartz tube for 30 min.
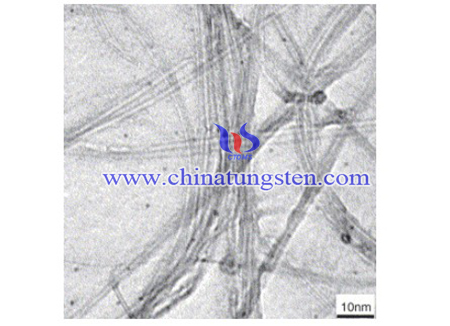
The catalyst was placed into the quartz tube. The furnace was first heated to 850 ∘C at the speed of 10 ∘C/min in an Ar atmosphere, and then a mixture of CH4 (45 ml/min) and Ar (150 ml/min) was introduced into the reactor, which was maintained at the same temperature for the production of SWCNTs. After 30 min reaction time, the reactor was cooled to room temperature in an Ar atmosphere. The products were dipped into hydrochloric acid, which can remove the MgO and most of the catalyst. The products were then rinsed with deionized water until the pH of the filtrate became neutral. The sample was then dried at 200 ∘C for 3 h. Purified SWCNTs samples were obtained in this manner.
The Raman spectra of the as-grown SWCNTs were recorded by a microprobe Raman system (RENISHAW H13325 spectrophotometer), and the excitation line was at 514.5 nm from an Ar-ion laser. Transmission electron microscopy (TEM) observations were performed at 100 kV with H-600 TEM made by the HITACHI Corporation.
In conclusion, the analysis showed that the W1–Co5 catalyst is efficient for the synthesis of SWCNTs. The diameter distribution of SWCNTs was estimated to range between 0.72–1.64 nm. When the molar ratios of W:MgO and Co:MgO in the catalysts are more than 2:100 and 5:100, respectively, the amorphous carbon content or defect concentrations of the SWCNTs increased with the increase of the quantity of metal in the catalysts.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com