The Preparation Method of the Tungsten Disulfide Nanoparticle
- Details
- Category: Tungsten Information
- Published on Sunday, 28 February 2021 23:00
There are many preparation methods of tungsten disulfide nanoparticles. Tungsten disulfide is two-dimensional material with layered structure and has excellent semiconductivity and diamagnetism. It is insoluble in acid, alkali and alcohol, and has certain reducibility, which can react with aqua regia, nitric acid and hot concentrated sulfuric acid, and is able to be oxidized to WO3 in air and oxygen atmosphere.
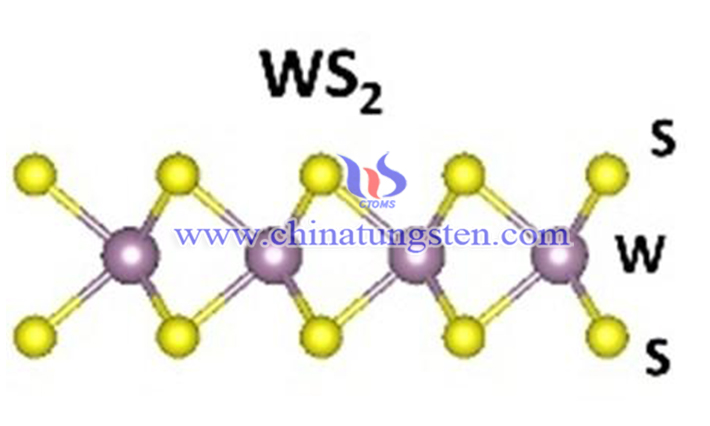
The structure of tungsten disulfide is similar to that of molybdenum disulfide, the preparation is different. Molybdenum disulfide can be prepared by flotation from natural molybdenum ore, but tungsten disulfide can not be obtained from natural ore, only can be prepared by chemical method. Due to its special nanostructure tungsten disulfide nanomaterial is one of the hot new functional materials developed by researchers at home and abroad. How to develop efficient, green and cost saving tungsten disulfide nanomaterials is the study hot.

Currently the methods of tungsten disulfide nanoparticles generally have physical preparation and chemical methods.
1.0 Physical preparation
The physical preparation is to pulverize and refine the massive tungsten disulfide to get nano-sized tungsten disulfide particles by physical methods such as ball milling, magnetic, electric treatment, ultrasonic treatment etc. Firstly the method is to synthesize high-purity tungsten disulfide bulk material in great numbers, and the first is to synthesize it by chemical method. The following operation is simple, However, it is difficult to prepare high-purity and monodisperse nanoparticles due to high requirement of equipment and the difficulty of controlling the particle size uniformity, which is not good for large-scale industrial application.
2.0 Chemical preparation
Usually the chemical preparation is to prepare the nanoparticles by chemical reaction using chemical substances as raw material. When the disulfide nanoparticle of tungsten is formed, some ways are used to control the growth of the nanoparticles to prevent the agglomeration of the nanoparticles and keep the nano size. Chemical methods generally include deposition, liquid phase, hydrothermal and surface modification and so on. It is easier to control the size of the particles and get monodisperse nanoparticles by chemical method. However, compared with physical method, the technique is more complex. There are many ways to prepare the nanoparticles, currently the large-scale preparation of nanoparticles is made by calcination decomposition method based on ammonium tetrathiotungstate . The main disadvantage of this method is to prepare tungsten disulfide by pyrolyzing ammonium tetrathiotungstate, it generates colorless, highly toxic and acidic by-product H2S hydrogen gas during preparation process, which not only does harm to operators, but pollutes the environment.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com