Gas–Solid Synthesis of Tungsten Carbide from Ammonium Paratungstate at Low Temperature
- Details
- Category: Tungsten Information
- Published on Thursday, 31 December 2020 16:49
Tungsten carbide (WC) has been widely applied as integral composition of hardmetal, or cemented carbide. Cemented carbides are composite materials consisting of a hard phase (WC) and a metal, usually Co, which binds the WC grains. The composition of the material (amount of Co) mostly influences their mechanical properties. The greater the Co content the tougher and softer the material is. Hardmetal is used for cutting, drilling and wear resistant pieces.
Tungsten carbide used for producing hardmetal is conventionally synthesized by heating a mixture of metallic tungsten powder and carbon black in a graphite furnace under a flowing hydrogen atmosphere.Fine grained alloys exhibit higher hardness than coarser grained ones of the same composition, at the same toughness level. The fine grained alloys (<0.5 μm) are used for producing micro drills used to drill printed circuits boards in the electronics industry. However, the traditional method to produce WC has the drawbacks of high temperature, longer production period, and it is difficult to produce fine grained WC.
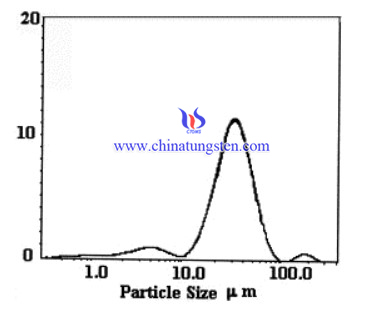
A gas–solid synthesis method of WC using ammonium paratungstate and tungsten blue oxide as tungsten source has been performed. It has low temperatures, ∼850°C, and shorter production periods.
The experimental procedures are as follows:
Ammonium paratungstate (APT) and tungsten blue oxide (TBO) are used as the tungsten sources. APT is used as received. TBO is prepared by heating APT, after milling it to reduce its particle size, at 600°C for 2 h under N2 flow. CH4 (99.95%) and H2 (99.9995%) are used as the carbon source and reducer.
The carburization reaction is carried out in a horizontal alumina fixed bed reactor. A boat with 3 g of APT or TBO is placed into the reactor. The temperature is raised at 5°C min−1 up to 850°C for APT and 820°C for TBO. The soaking time is 2 h in both cases. During heating and isotherm, a gaseous mixture CH4+H2 flows through the reactor at a rate of 20 l h−1. The composition of the mixture (95% H2–5% CH4) is controlled by two Brooks 5850TR flowmeters.

APT and TBO were characterized by X-ray diffraction. TG/DTA of APT in argon was carried out to show how it decomposes. The carburized products of APT and TBO were characterized by XRD, SEM and laser scattering particle size measurement.
In summary, the carburization of TBO should proceed through the reduction of different intermediate tungsten oxides up to tungsten metal by H2 and then the carburization of W by methane. The direct carburization of APT starts with its decomposition, in which H2O and NH3 evaporate, to form a tungsten oxide. Next, the reaction follows the same steps as the carburization of TBO. The WC particles have roughly the same size and shape as the APT and TBO particles. They consist of WC crystallites <1 μm.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com