Why Tungsten Trioxide Is A Promising Photocatalyst Material?
- Details
- Category: Tungsten Information
- Published on Wednesday, 08 August 2018 19:38
Tungsten trioxide (WO3) is one of the earlier studied photocatalysts. There are other promising photocatalysts such as titanium dioxide, iron oxide and so on. But what makes tungsten trioxide one of the earliest materials studied in the field of phtocatalysts? This is due to the light stability of tungsten trioxide, a wide range of sources, and it is environmental friendly. It is no wonder that it will be favored by researchers.
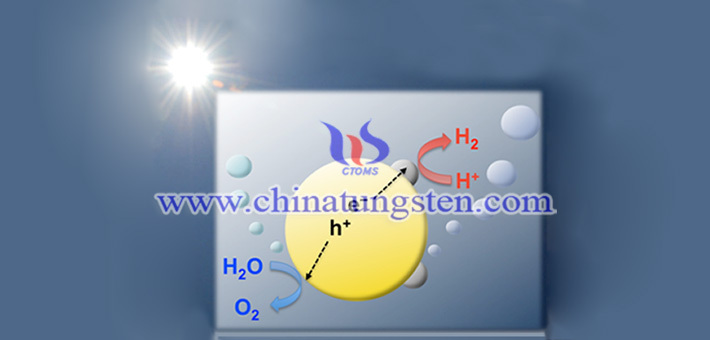
So, what applications does tungsten trioxide have in the field of photocatalysis? Tungsten trioxide has broad application prospects in the fields of photocatalyst decomposition of water to produce hydrogen, photoelectrochemical cells and pollutant removal. Photocatalytic technology - is considered the most effective way to control environmental pollution! It is a method that only needs to use solar energy to effectively remove pollutants with high toxicity, low concentration and difficult treatment, and does not cause other additional effects.
The lower position of the WO3 conduction band limits the effective separation of photogenerated carriers. Naturally, researchers have made a lot of efforts to overcome this shortcoming, but research on tungsten trioxide photocatalyst still has a long way to go.
In the early stage, the wide-bandgap semiconductor with relatively obvious photocatalytic performance was the main research object of photocatalyst, such as TiO2. So, how does the research scope of the catalyst extend to tungsten trioxide? With the deepening of research, it has been found that semiconductors such as tungsten trioxide also have photocatalytic activity, and it is important to be able to respond to longer wavelength light, which increases the utilization of light, thereby expanding the scope of photocatalyst.
In 1953, Markha et al. studied the kinetic behavior of hydrogen peroxide on the surface of zinc oxide (ZnO) under light. It was found that benzene can be oxidized by oxidation to a peroxidized organic substance under illumination. In 1972, Fujishima et al. found that titanium dioxide (TiO2) photoelectrodes can generate photogenerated electrons and holes under the irradiation of light with a wavelength of less than 415 nm, and can react with water to generate hydrogen and oxygen. Many scientists have conducted extensive research on this phenomenon. In 1976, Carey et al found that titanium dioxide can decompose polychlorinated biphenyls under ultraviolet light. The following year, Frank et al. conducted in-depth research on photocatalytic oxidation of cyanide ions and sulfites. Common to all of these groundbreaking studies is the association of light and semiconductors to accomplish energy conversion and use it for environmental governance. Since then, a new method of using solar energy for pollution control has attracted people's attention, and there has been research on the future of tungsten trioxide in the field of photocatalysis.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com