Ammonium Paratungstate Evaporation Crystallization
- Details
- Category: Tungsten Information
- Published on Friday, 02 February 2018 16:04
Preparation of ammonium paratungstate by evaporative crystallization is the main method of industrial production of tungsten products. Ammonium paratungstate evaporation crystallization principle is through the use of ammonium tungstate solution itself special nature: ammonium tungstate solution contains more free ammonium, free ammonia is easy to volatilize, ammonium tungstate is very unstable.
Ammonium paratungstate evaporation crystallization is through the temperature, evaporation and stirring ammonium tungstate solution. As ammonia and water continue to volatilize, the pH of the solution also decreases, resulting in the continuous generation of ammonium paratungstate. As the ammonium paratungstate solubility becomes smaller, making ammonium paratungstate constant precipitation from the solution. At the same time, the solution of ammonium paratungstate concentration from unsaturated to saturated, so that the solution into the metastable state. As evaporation continues, nuclei are induced to nucleate in a reducing ammonia atmosphere, and nuclei then grow to crystals of a certain size and shape. The chemical reaction process of preparing APT by evaporating crystallization from ammonium tungstate solution is essentially the process of losing ammonia.
The ammonium paratungstate evaporation crystallization method of chemical reaction is:
12(NH4)2WO4→ 5(NH4)2O·12WO3 ·n H2O +14NH3↑+(7-n)H2O
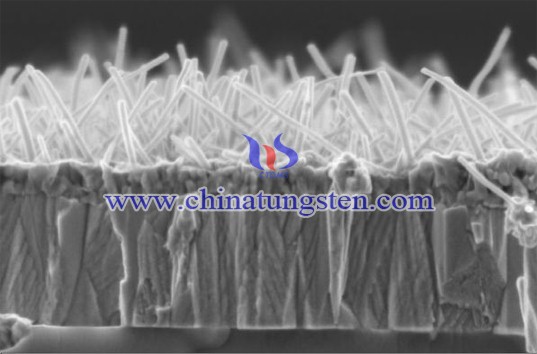
In the above reaction formula, the amount n of ammonium paratungstate crystal water depends on the reaction temperature of evaporation crystallization. When the reaction crystallization temperature is higher than 50 ℃, the value of n = 5, ammonium paratungstate product is flaky crystal; when the reaction crystallization temperature is lower than 50 ℃, the value of n = 11, ammonium paratungstate product is white needle crystal. At the same time, the reaction crystallization process should control the crystallization rate of tungsten to control the crystallization rate of impurities increased.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com