Carbon Dioxide Preparing Pyrochlor Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Tuesday, 02 February 2016 16:32
- Written by qiongyao
- Hits: 238
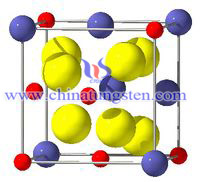 Pyrochlore type structure is also known as yellow-green stone structure or pyrochlore structure. Formula A2B2O7, (BO6) octahedra are connected by common oxygen vertex along the three-dimensional framework that is formed. (BO6) slightly distorted octahedral shape; the ion is located in the gap BO6 skeleton. Many complex oxides having such structure, such as coke cadmium ,niobate Cd2Nb2O7, tantalum acid pyrophosphate cadmium Cd2Ta2O7, coke lead niobate Pb2Nb2O7, coke cerium zirconate Ce2Zr2O7 like. Many of these oxides are important ferroelectrics, which are widely used in electronic ceramics.
Pyrochlore type structure is also known as yellow-green stone structure or pyrochlore structure. Formula A2B2O7, (BO6) octahedra are connected by common oxygen vertex along the three-dimensional framework that is formed. (BO6) slightly distorted octahedral shape; the ion is located in the gap BO6 skeleton. Many complex oxides having such structure, such as coke cadmium ,niobate Cd2Nb2O7, tantalum acid pyrophosphate cadmium Cd2Ta2O7, coke lead niobate Pb2Nb2O7, coke cerium zirconate Ce2Zr2O7 like. Many of these oxides are important ferroelectrics, which are widely used in electronic ceramics.
Since the pH of the solution is important for the preparation of tungsten trioxide pyrochlore type process and in the alkaline environment of the powder can be prepared, therefore, the following describes carbon dioxide as an additive to prepare pyrochlore type tungsten trioxide. Taking carbon dioxide as additive to prepare pyrochlore-type structure of the tungsten trioxide. First, preparing 100g / L of sodium tungstate solution, taking the carbon dioxide into the solution until the pH value of the sodium tungstate solution decreases to 7.34, and then placing the carbon dioxide treatment after the sodium tungstate solution in an autoclave 100mL at 200 ℃, reflecting it at 260 ℃ conditions about 24h, removing the solution, and washing the resulting product with deionized water 2-3 times, drying it t at 60 ℃, finishing pyrochlor tungsten trioxide.
As we can see from the entire manufacturing process, though the carbon dioxide presents in the solution, the entire system or hot water is alkaline under alkaline conditions, it is also can be prepared pyrochlore type tungsten trioxide.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com