"Lasagne" Made of Molybdenum Disulfide and Molybdenum Diselenide Keeps Heat Out
- Details
- Category: Tungsten's News
- Published on Sunday, 07 November 2021 22:40
- Written by Caodan
- Hits: 657
A team led by Professor Kazuhiro Yanagi at Tokyo Metropolitan University has been working on ways to produce and process ultra-thin layers of a class of materials known as transition metal dichloride. Here, they have taken single-atom-thick layers of molybdenum disulfide and molybdenum diselenide and stacked them together in four layers (4L films).
Tokyo Metropolitan University’s mission is “to pursue the vision of an ideal human society in a metropolis.” Based on this mission, their basic philosophy is to give instruction and conduct research in a broad range of knowledge and in highly specialized sciences, and then, through collaboration with industry and educational and research institutions, etc., to produce education and research results that are relevant to large cities, to nurture students rich in humanity and creativity, and to contribute to the improvement and development of human society.
Different levels of heat transfer were found in the layers formed by chemical vapor deposition, annealed weakly bonded layers, weakly bonded layers, and alternating layers consisting of two different materials.
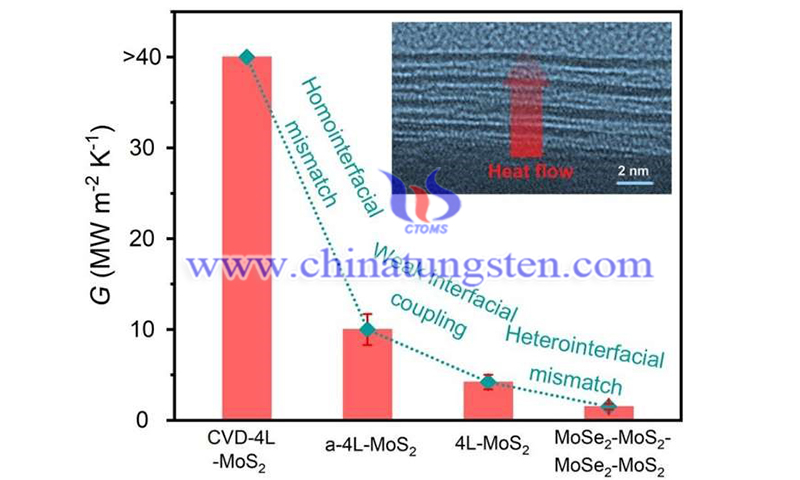
Researchers from Tokyo Metropolitan University have discovered new ways to control how heat flows through thin materials by stacking atomically thin layers into van der Waals heterogeneous structures. By comparing different stacks of different materials, or even the same material after heat treatment, they found that weak coupling and mismatching between layers helped to significantly reduce heat transfer. Their findings hold promise for sensitive control of heat flow at the nanoscale in thermoelectric devices.
Heat is everywhere, and it flows. Heat in the wrong place can also cause damage. For example, because microchips generate more heat than they can carry away when performing intensive computing tasks, they can overheat electronics. This can damage or severely reduce the lifetime of electronic devices, making the control of heat flow at the nanoscale a pressing issue for modern society.
These layers can be coupled together in different ways. The research team transferred large single-atom sheets in a unique, gentle way, allowing them to create stacks of layers bound together by van der Waals forces. They can also be forcibly bonded by more conventional techniques, particularly chemical vapor deposition (CVD). This creates many variations on how the isolated layers are put together and may control how heat passes through them.
By using a special coating technique, they were able to detect how miniscule amounts of heat flowed past these stacks with rather good accuracy. Firstly, they found that layers strongly bound by CVD let through significantly more heat than their loosely bound counterparts. This effect could be partially reversed by annealing weakly held layers, making the binding stronger and improving upon the transport of heat.
Furthermore, they compared stacks of four molybdenum sulfide layers to a "lasagna"-like structure made of alternating layers of molybdenum disulfide and molybdenum selenide. Such heterostructures had an artificial structural mismatch between adjacent layers of atoms which led to significantly lower levels of heat transfer, more than 10 times less than with strongly bound layers.
The team's findings not only demonstrate a new technological development but provide general design rules on how to control heat flow on the nanoscale, whether you want more or less flow. These insights will lead to the development of ultra-thin, ultra-light insulators and new thermoelectric materials where heat may be efficiently directed and converted into electricity.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com