WO3 Acts as Electrode Materials for ECs, ECDs and ESDs
- Details
- Category: Tungsten's News
- Published on Sunday, 15 January 2023 00:05
- Written by Caodan
- Hits: 739
Electrochromic materials (EC), electrochromic devices (ECDs) and energy storage devices (ESDs) own similar device structures and operating principles, and transition metal oxides, such as MoO3, MnO2 and WO3, can be used as electrode materials for these devices. EC materials can change their optical parameters, including reflectance, refractive index, transmittance and emissivity, when a relatively low voltage (even less than 1 V) or electric field is applied, and the process is reversible when the polarity of the voltage or electric field is reversed. Because of this special property, ECDs are widely used in smart windows, anti-glare mirrors, display applications, and in aerospace and military applications.
In particular, since buildings account for 40% of global energy consumption, when they are used as smart windows, they can save significant amounts of energy due to their adjustable transmittance to sunlight. It has also been reported that EC smart windows outperform photovoltaic devices in terms of energy savings. In addition, ECDs can be considered as transparent efficient energy storage devices (ESDs) given that the discoloration process of ECDs is also related to the insertion and extraction of ions.
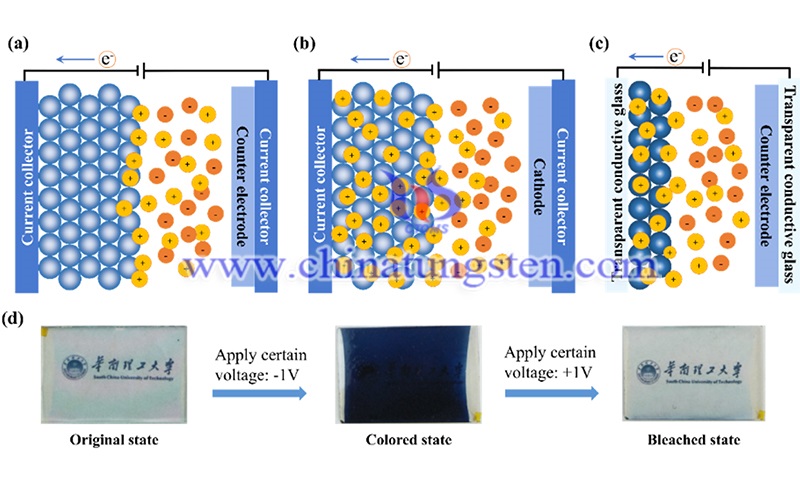
The above three devices have similar device structures and operating principles. In addition, many transition metal oxides, such as MoO3, MnO2 and WO3, can be used as electrode materials for these devices. Among them, tungsten oxide has a large energy storage capacity, which enables it to play an electrode role in ESD (including SC and LIB), and it is also the most widely studied material in the field of EC.
When used as an electrode for SC, its theoretical specific capacity of 1112 F.g-1 is much higher than that of carbon electrode materials commonly used for double-layer capacitors, due to the fact that the valence of W can vary between +2 and +6, and when used as an anode for LIBs, its theoretical specific capacity of 693 mA h g-1 is almost twice that of graphite. In addition, they offer other advantages, including high density, low cost, environmental friendliness and non-toxicity. As in the field of electrical conductivity, Deb first discovered it in tungsten oxide in the 1960s. Tungsten oxides are favored for their short switching times, impressive color changes and electrochemical stability.
Considering that ESD and ECD have some correlation, tungsten oxide electrochromic energy storage devices, either electrochromic supercapacitors (ECSC) or electrochromic batteries (ECB), have also attracted attention. We can get the information of its working state directly from the color signal, which brings us great convenience and safety, or we can think of it as a transparent battery, which makes good use of the energy stored in it and reduces the power consumption. In addition, these bifunctional devices can have more possibilities to realize self-powered systems by integrating other parts, such as solar cells.
In addition, it can output electrical energy generated by solar cells for efficient use of energy. Given the wide range of uses of tungsten oxide materials (Figure 1), there are many studies on them and some researchers summarize their development in specific areas such as electrochromic aspects, photocatalyst aspects, and sensors.

Reference: Han W, Shi Q, Hu R. Advances in electrochemical energy devices constructed with tungsten oxide-based nanomaterials[J]. Nanomaterials, 2021, 11(3): 692.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com





 sales@chinatungsten.com
sales@chinatungsten.com