Chitosan Combined with Ammonium Paratungstate as Anticorrosive Coatings
- Details
- Category: Tungsten Information
- Published on Tuesday, 10 November 2020 12:10
Chitosan (Chit) is a non-toxic derivative of chitin, namely the β-(1,4)- linked polysaccharide of D-glucosamine. Application of chitosan coatings on substrates by the dip coating technique was proved to be highly effective in corrosion prevention by producing thin layers with controlled characteristics. The transparency of chitosan coatings also constitutes an advantage as it does not change the appearance of the underlying substrate.
The loose gel-structure of chitosan can act as a container for corrosion inhibitors or can be crosslinked (either covalent or ionic) to modify its structure, reducing permeability and swelling. Ammonium paratungstate (APT) is considered as a potential material to crosslink to Chit.
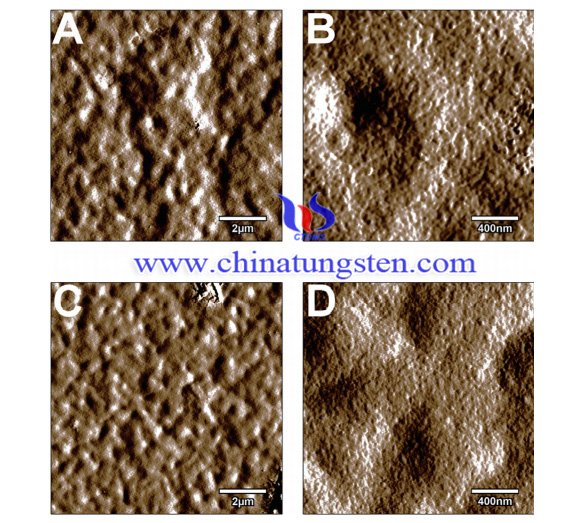
This study aims to improve the modest barrier properties of chitosan coatings by impregnating them with ammonium paratungstate, which can act both as an ionic crosslinker and as a corrosion inhibitor to reduce the corrosion rate of zinc substrates.
The preparation process are as follows:
Quartz, glass, and zinc plates were used for the chitosan coatings’ characterization. Prior to coating, the glass and quartz slides were cleaned by an aqueous detergent solution, 10% V/V H2SO4 solution, isopropyl alcohol, distilled water, and deionized distilled water (18.2 MΩ·cm, purified with Millipore Simplicity 185 filtration system). Zinc samples were prepared for dip coating by polishing with abrasive paper (1200, 2000, 2500), Al2O3, 2 × 5-minute ultrasonication (to remove any particle residue from the polishing) and treatment with 0.1 M HCl solution (5 s) to clean the surface of any left-over zincoxide.
For dip coating, 1 wt/% chitosan was dissolved in a 1 w/w% aqueous acetic acid solution by mixing the chitosan until full dissolution. Insoluble chitosan residue (due to incomplete deacetylation) was then removed by a 30-minute centrifugation step at 4000 rpm [8]. The coatings were prepared by dip coating with a withdrawal speed of 5 cm/min [20]. Samples were dried for 24 h at room temperature. After drying, the chitosan-coated glass, quartz and zinc samples were impregnated from aqueous PTA solutions. The impregnation was realized by immersing the samples with a speed of 1 cm/min and then keeping them for 15 min in the impregnating solution, followed by immediate withdrawal. Finally, the impregnated samples (Chit-APT) were rinsed with distilled water and dried at room temperature. The stability of the Chit-APT coatings was investigated by immersion in distilled water. The samples were withdrawn and dried 2, 4, 18 and 24 h after immersion (to record absorbance spectra) before being returned into the distilled water.
In conclusion, no obvious anticorrosive properties have showed in the native chitosan, while the Chit-APT coating shows an excellent anticorrosive efficiency of 97.91%.
The reduction of the polarization resistance and the change of the fitted equivalent circuits point out the leaching of the Chit combined with APT as anticorrosive coatings over time, which reduces the protective properties of the chitosan layer. Nevertheless, the Chit-APT coating can be an effective temporary protective barrier for zinc, which is removable on demand by scrubbing with mild acidic solutions, leaving the underlying metal unharmed. These coatings can be implemented in the temporary protection of zinc parts during transport and can be removed to allow further processing of the substrates.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com