Rare Earth Could Be Replaced by Common Elements Compounds
- Details
- Category: Tungsten's News
- Published on Tuesday, 16 July 2019 11:52
Rare earth is indispensable raw materials in various fields. Recently, the University of Michigan team has developed a technology that combines the ordinary elements into common elements compounds that can replace rare earth metals and has the opportunity to reduce the dependence of industries on rare earth elements.
Rare earth is the combination of 17 chemical elements such as scandium, yttrium and lanthanides series of the sub-group III elements, specifically including 15 elements of the lanthanide series: La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, and two elements closely related to the lanthanide: Sc and Y.
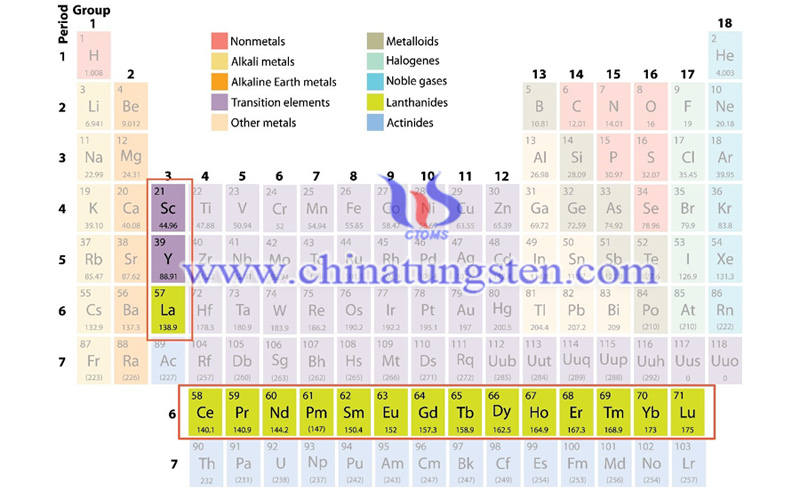
All kinds of applications such as solar panels, wind turbines, electronic equipment, machinery, electric vehicles, mobile phones, and LED bulbs that illuminate indoors are using expensive rare earth metals. At present, solar panels have high conversion rate is also attributed to rare earth metals such as indium, gallium, selenium, etc.; or tungsten carbide (WC) hard alloys, which are often used in the aerospace, metalworking, mining and construction industries because of its high melting point.
Nowadays, nearly 80% of the world's rare earths are supplied from China. Besides, it is difficult to effectively recycle rare earth metals at present, and the application of various industries depends on its raw materials that may be exhausted within 10 to 20 years, which make many countries feel insecure.
Therefore, scientists from all over the world are committed to the development of common elements compounds that can replace rare earth, including Honda, Samsung, and other companies that have developed recycling methods. Recently, a team of the University of Michigan, the University of Lorraine, and the University of Canterbury used molecular beam epitaxy (MBE) technology to combine ordinary elements with cheaper prices and wealthier crust content into II-IV-V group compounds successfully. It can replace the rare earth elements that are often found in the three-five (III-V) group.
II-IV-V group compounds have similar properties to rare earth elements. For example, zinc-tin-nitrogen compounds can be "tuned" to absorb sunlight of different wavelengths, which means rare earth used in thin-film solar panels, LED lights, television displays, and mobile phone screens have opportunities to be replaced. In addition, new compounds combined with specific types of elements, due to the array of the elements, can also make the material sensitive to light of a specific wavelength, and can produce light-emitting diodes that emit light of a specific color.
Researches on common elements compounds that replace rare earth may change the development of the rare earth market in the future. The next phase of the research team will examine the efficacy of various new compounds in detail and test the different functions that various nanostructures may bring. The new paper was published in the journal Physical Review Letters (PRL).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com