MIL-loaded Phosphotungstic Acid
- Details
- Category: Tungsten Information
- Published on Tuesday, 03 September 2019 23:26
Photocatalytic oxidation technology has good selectivity, can react at room temperature and pressure under mild conditions, and can degrade organic pollutants into non-toxic and harmless inorganic substances. It is a green technology with important application prospects in the field of energy and environment. In recent years, more and more reports have been reported on photocatalytic oxidation of organic wastewater. Semiconductor materials used are also various, such as titanium dioxide, zinc oxide, Fe2O3, CdS, WO3, etc. The photocatalytic stability of WO3 is good, but its activity is relatively small.
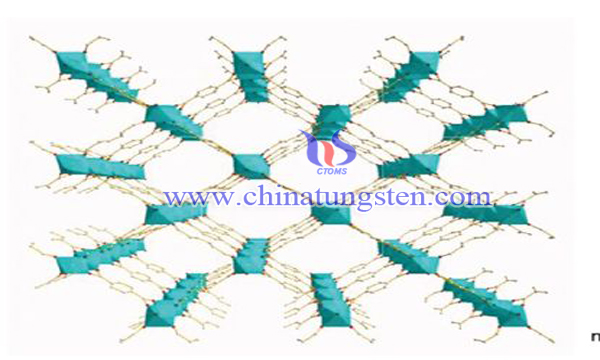
The tungsten-phosphorus heteropoly acid with tungsten as the precursor has the ideal photocatalytic oxidation characteristics. For example, the molecular radius of the polyoxometal complex is approximately 1 nm, which can be regarded as a quantum-scale semiconductor material, and its standard redox state of the excited state. The potential is about 2.63V, which can also effectively degrade organic pollutants; the toxicity is extremely low, and it does not pollute the environment; the activity is high, the reaction conditions are mild, and the equipment is not corroded.
However, soluble heteropoly acids have some problems, such as difficult separation and recycling, high pH requirement and low quantum efficiency. Therefore, immobilization of heteropoly acids has become a research hotspot. At present, MIL series materials have become one of the most widely studied metal-organic framework materials in the field of heterogeneous catalysis. The material is a crystalline material with porous structure formed by self-assembly of rigid polydentate carboxylic acid ligands, such as chromium, iron, aluminium or vanadium, and terephthalic acid or pyromellitic acid. It has excellent performance in adsorption, separation and catalysis. MIL-loaded phosphotungstic acid undergoes the following processes:
0.498g terephthalic acid was dispersed in 15mLDMF by ultrasound, then 0.8109g FeCl3·6H2O and 0.162g phosphotungstic acid were added successively, stirred by magnetic force for 15 minutes, reacted in a high-pressure reactor lined with polytetrafluoroethylene (PTFE) at 150 ℃ for 5 hours, cooled to room temperature, centrifuged, washed with water and ethanol, and finally dried in vacuum at 60 ℃ for 24 hours.- 53 (Fe).
Organic dye Rhodamine B was selected as probe molecule to prepare photocatalyst. The rhodamine B dye wastewater with 20 mg/L concentration of 25 mL was prepared. A certain amount of photocatalyst was added to the wastewater and magnetic stirring was conducted at room temperature. The organic pollutants in the water were degraded under the irradiation of a LED lamp with 10 cm, 20 W and 450 nm above the water surface. The absorbency of the water samples was measured at 554 nm after filtering at a certain interval. Finally, the degradation rate of rhodamine B was calculated.
The degradation rate of Rhodamine B was 97.57% after 120 min degradation by adding 5 mg MIL loaded phosphotungstic acid composite into the wastewater of Rhodamine B. Because the molecule of phosphotungstic acid is larger than the pore size of MIL (Fe), it is difficult to enter the water, so it is easy to centrifuge and recycle, environmentally friendly and no secondary pollution.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com