Tungsten Disulfide Nanorods Preparation by Hydrothermal Synthesis
- Details
- Category: Tungsten Information
- Published on Sunday, 25 August 2019 10:38
Tungsten disulfide is a typical layered transition metal disulfide with van der Waals force interaction between layers. Nanostructured WS2 can be used in solar cells and field-effect transistors because of its enhanced catalytic performance, excellent optical conductivity and excellent friction properties.
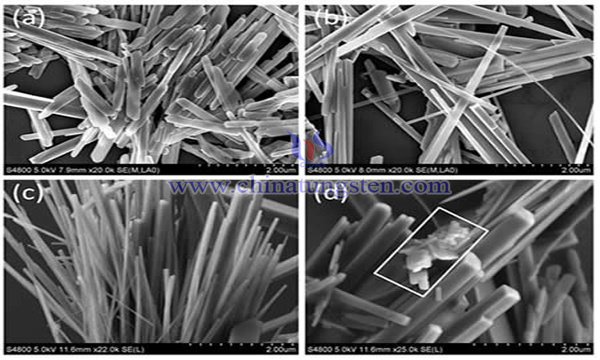
WS2 has a layered structure similar to graphite lamellae, with strong covalent bonds in the layer and weak van der Waals force between the layers. The layers and layers are easily peeled off, and have good anisotropy and low friction coefficient. Some scholars have reported a preparation method of tungsten disulfide nanorods. The precursors were prepared by chemical precipitation-desulfurization method and ball milling-desulfurization method, i.e. ball milling of WS2 and S powders, and then WS2 nanorods were prepared in a high-pressure reactor at 240 C for 24 h. This process usually requires the preparation of precursors. The process is complex, and WS2 nanorods have uneven particle size and poor morphology control, which affects their applications in tribology, photochemistry and other fields.
In order to solve the above technical problems, some scholars have proposed a hydrothermal synthesis method to prepare tungsten disulfide nanorods, including the following steps:
A.Water and ethanol were mixed into mixed solvents according to the volume ratio of water to ethanol=7:13, and 30 mg tungsten disulfide powder was added into 20 ml of the above mixed solvents for hydrothermal synthesis at 200 ℃ for 10 h.
B.Tungsten disulfide nanorods were prepared by centrifuging the reacted solution at 500 rpm for 30 min at 400 W power for 1 h.
Among them, (NH4)2WO4 is tungsten source, CS(NH2)2 is sulfur source, (NH4)2WO4 reacts with CS(NH2)2 to produce ammonium thiotungstate, and NH2H·HCl reacts with ammonium thiotungstate as reducing agent to obtain tungsten disulfide.
The above process uses NH2OH·HCl as a reducing agent, and in the reaction, it is oxidized into a gas such as nitrogen, water and nitrogen monoxide, which does not bring impurities to the reaction, improves the purity of the final product, and obtains tungsten disulfide. The particle size and morphology of the nanorods can be controlled, the layered structure is obvious, and the specific surface area and friction properties are high.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com