Cubic Hydrogen Tungsten Bronze Crystal Structure
- Details
- Category: Tungsten Information
- Published on Wednesday, 18 May 2016 18:17
- Written by xinyi
- Hits: 205
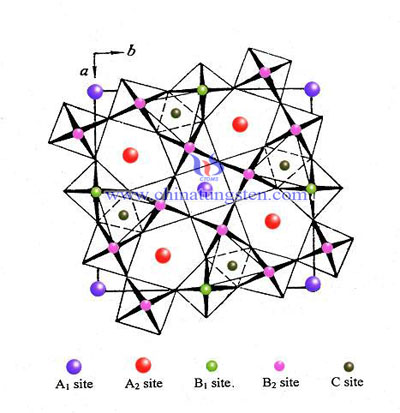
The structure of D0.53WO3 has been determined by a room temperature powder neutron diffraction study. The unit cell is body centered cubic (Im3) and contains 8 formula weights (a = 7.562 ± 0.002 Å). The average cell content was refined by a least squares method based on peak intensities. The tungsten atoms lie on the special sites of the perovskite structure and are surrounded by nearly regular octahedrons of oxygen atoms with the latter displaced from the normal perovskite positions along 〈110〉. The deuterium atoms constitute statistically distributed -OD bonds which are directed towards the nearest oxygen atom of a neighboring octahedron. The analogous hydrogen compound(cubic hydrogen tungsten bronze) is best described as a nonstoichiometric oxide hydroxide WO3−x(OH)x based on a distorted ReO3 structure. The crystal structure is closely related to that of In(OH)3 and Sc(OH)3.
In order to understand the slow stages of the catalytic reduction of WO3 to hydrogen tungsten bronzes HxWO3, the mobility of hydrogen in the latter has been studied by NMR spectroscopy between −50 and −195 °C. Values of the spin-lattice relaxation time T1 plotted vs. the inverse temperature show a minimum indicating one main relaxation process, namely that due to diffusion. The corresponding calculated diffusion coefficient of H in HxWO3 is very high. Thus transport of H in HxWO3 cannot be the rate determining step in the catalytic reduction of WO3 Rather, that step is suggested to be the penetration of the reducing species below the surface of WO3 into the lattice.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com