Tactical Gloves with Cut-Resistant Tungsten Wire
- Details
- Category: Tungsten Information
- Published on Monday, 07 April 2025 18:35
- Written by Zhenghua
- Hits: 171
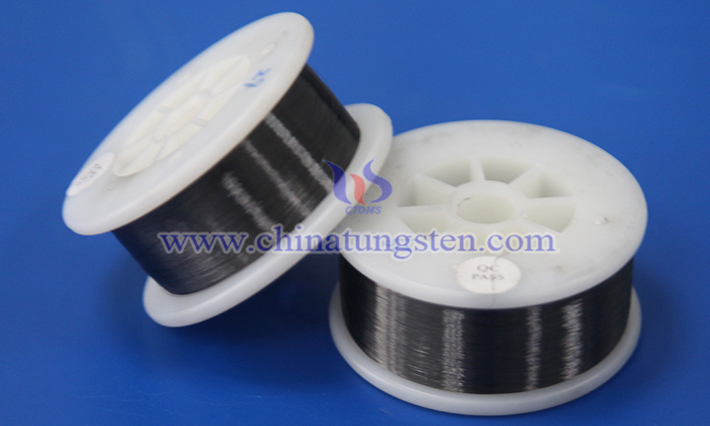
Tactical gloves, designed specifically for military, law enforcement, and outdoor activities, prioritize both protection and flexibility. In recent years, advancements in material science have introduced cut-resistant tungsten wire into the design of tactical gloves, bringing new possibilities to traditional protective capabilities.
Stab-Proof Vests Using Cut-Resistant Tungsten Wire
- Details
- Category: Tungsten Information
- Published on Monday, 07 April 2025 18:33
- Written by Zhenghua
- Hits: 181
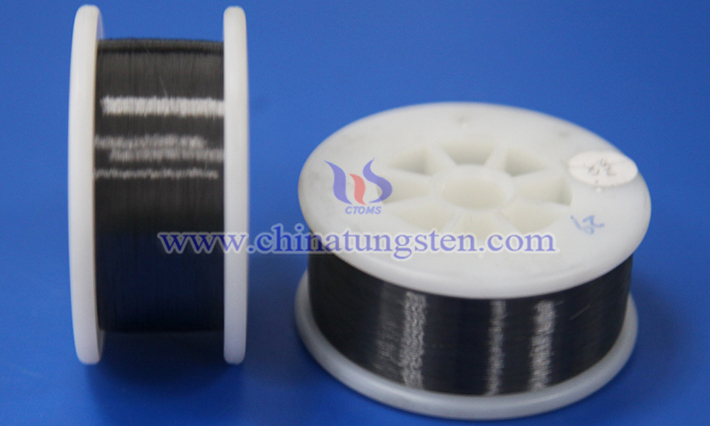
Stab-proof vests, as a vital piece of personal protective equipment, are widely used by law enforcement officers, military personnel, security guards, and individuals in high-risk occupations. Their primary function is to defend against stabbing, slashing, and chopping attacks from sharp implements such as knives and daggers.
Read more: Stab-Proof Vests Using Cut-Resistant Tungsten Wire
Protective Gloves with Cut-Resistant Tungsten Wire
- Details
- Category: Tungsten Information
- Published on Monday, 07 April 2025 18:27
- Written by Zhenghua
- Hits: 161

In modern industry and daily life, the importance of safety equipment is becoming increasingly evident. Particularly in scenarios involving sharp objects or high-risk operations, protective gloves have emerged as critical gear for safeguarding hands from injury. In recent years, a novel material—cut-resistant tungsten wire—has gained traction in the manufacturing of protective gloves due to its exceptional performance, offering users a higher level of safety assurance.
Read more: Protective Gloves with Cut-Resistant Tungsten Wire
Cut-Resistant Tungsten Wire for Protective Clothing
- Details
- Category: Tungsten Information
- Published on Monday, 07 April 2025 18:30
- Written by Zhenghua
- Hits: 180

Cut-resistant tungsten wire is a material characterized by high strength, high hardness, and excellent heat resistance. Due to its outstanding physical properties, it is frequently utilized in the production of protective clothing, particularly for gear designed to withstand cutting, piercing, or high-temperature environments.
Read more: Cut-Resistant Tungsten Wire for Protective Clothing
What Is the Relationship Between the Crystal Structure and Properties of Yellow Tungsten Oxide?
- Details
- Category: Tungsten Information
- Published on Saturday, 05 April 2025 11:27
- Written by Xiaoting
- Hits: 174

Yellow tungsten oxide (WO₃) produced by CTIA GROUP LTD leverages its unique crystal structure to exhibit exceptional chemical and physical properties, making it a vital material across various fields. Typically adopting an orthorhombic crystal structure, WO₃ features oxygen atoms arranged in a near-octahedral configuration around tungsten atoms, forming a stable three-dimensional network. This arrangement endows yellow tungsten oxide with remarkable stability, electrical properties, and optical characteristics, enabling its widespread use in electronic devices, energy storage systems, and glass applications.





 sales@chinatungsten.com
sales@chinatungsten.com