Tungsten Carbide Equipment Solve Partial Wearing Problem
- Details
- Category: Tungsten's News
- Published on Monday, 22 August 2016 18:08
- Written by tang
- Hits: 577
Recently, the Yumen Oilfield Oil Production Plant oil duck children gorge team technical team leader Li Ning serious stare duck K1-10 wellsite conceal his face is delighted: "Since the use of this device, which I often need surgery well made no been ill, and now we can rest assured that in other to promote the use of the well. "
Duck children Gorge Reservoir Oil Production Plant complex geological structure, oil prone eccentric wear phenomenon. Previously, in order to prevent eccentric wear caused by oil bar broken off, oil wells team technical team to install anti-eccentric wear equipment. Employees also use the opportunity well underground work, from a downhole sucker rod section hoop, pump, tubing head thread and other downhole equipment to conduct a comprehensive inspection. However, anti-bias milling equipment still could not really solve the oil well pumping unit section hoop, threaded tubing head appeared eccentric wear problems. Especially in recent years duck children gorge horizontal wells increased, accounting for 70% of new drilling, pump hanging in 1900-2100 meters, displacement of up to 200 meters above the bottom, resulting in serious eccentric wear rod, using up more than 120 days will be break off occurs.
Since the beginning of this year, for the control of oil wells due to the occurrence of breaking off caused by multiple rounds lie well, duck child Gap oil technology group team counting finer benefits account. And repeated the repair and replacement of equipment, is bound to increase costs, affecting the normal production wells.
After then, the duck child Gap oil oil drilling teams to coordinate the Institute of plant and machinery, and the geological structure of the reservoir section hoop, tubing head material such as a comprehensive analysis of the carbide homemade device. Grindability in the well area installed in the underground ring segment added centralizer re-optimize the portfolio by carbide strength and good finish, reduced wear resistance. Duck child Gap oil production crew through an annular segment downhole added centralizer re-optimize the adjustment, prevent breaking off the hoop section, extending well exemption period.
Li Ning said: "We selected four sections hoop and tubing head screw serious eccentric wear wells may be used, the effects were good, especially the duck duck K1-10 and K1-15 well well, since after the equipment installed on the carbide never happened broken off. Tungsten carbide equipment not only solves the problem of partial wear well, but also reduces the cost of repair of oil wells. " Next, duck children gorge on oil production crew will be easy to promote the use of eccentric wear wells carbide devices.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Hydrogen Doped WO3 Plasmon Nanostructure
- Details
- Category: Tungsten's News
- Published on Thursday, 18 August 2016 11:36
- Written by chunyan
- Hits: 745
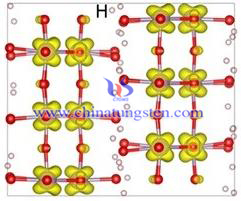 Since the importance of plasmon nanostructure in areas of nano photonics, biochemical sensing and medical diagnostics ect., manufacturing technology of plasma nanostructure has recently caused a lot focus for people researching. By controlling the size, shape and composition of plasma nanostructures, one can adjust its optical and electrical properties over a wide range. With the advent of nanoscience and nanotechnology, plasmon nanostructure has developed rapidly, the coherent oscillations of free electron has on the incident light will cause localized surface plasmon resonance (LSPR) in the structure; besides, this phenomenon is associated with optical near field zoom, and has been used in surface-enhanced Raman scattering (SERS), biological testing and energy storage and conversion and other aspects.
Since the importance of plasmon nanostructure in areas of nano photonics, biochemical sensing and medical diagnostics ect., manufacturing technology of plasma nanostructure has recently caused a lot focus for people researching. By controlling the size, shape and composition of plasma nanostructures, one can adjust its optical and electrical properties over a wide range. With the advent of nanoscience and nanotechnology, plasmon nanostructure has developed rapidly, the coherent oscillations of free electron has on the incident light will cause localized surface plasmon resonance (LSPR) in the structure; besides, this phenomenon is associated with optical near field zoom, and has been used in surface-enhanced Raman scattering (SERS), biological testing and energy storage and conversion and other aspects.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Metatungstate Price Affecting Factors
- Details
- Category: Tungsten's News
- Published on Tuesday, 16 August 2016 14:13
- Written by Cristina
- Hits: 756
Under the conditions of depressed manufacturing industry, tungsten market also enters normalization ordinary. Tungsten raw material capacity stays in the constant high level. With the application of tungsten waste, end market lowers its dependency on tungsten raw material. Cost of heavy mining is increasing, it results in the irreconcilable conflicts of tungsten market.
Ammonium metatungstate price affecting factors is mainly related to raw material price, below are the analysis of those factors:
1.Tungsten raw material producing capacity is relatively at high level, supply of tungsten concentrate and APT overdue actually demanding which results in the unbalance of supply-demand relationship, it affects tungsten raw material price directly.
2.Tungsten mine cost directly affects price, heavy mining based on state-owned enterprise has high cost; medium and small mine is of lower cost. So tungsten concentrate of low price is sometimes active in the market.
3.Enterprise property of tungsten mine has effects on price as well. More than 50% tungsten concentrate originated from state enterprise tungsten mine. State-owned enterprise actively supports market place. When the price is too low, they will refuse to sell the product to protect rare metal source.
4.Capacity of tungsten concentrate is relatively centralized, more than 55% is from eight major tungsten enterprises. Since last year, three conference of the enterprises also save the market in a certain degree.
5.Through out a year, tungsten market will go through the spring supply and summer break. After spring festival, there is once confused of market, most tungsten raw material companies are reluctant to sell and hold on to see the trend of market. It results in the market’s short supply and increases of price. Summer break is often in July and August, most European countries takes summer break and stops production. The demanding is rare during this period.

6.China Tungsten Industry Association and Ganzhou Tungsten Industry Association has effects on tungsten market as well. When the market is unstable, they will preserve interests of members.
7.Market guidance price of Ganzhou Tungsten Industry Association and China Minmetals Group publicized every month have effect on market price.
8.State Reserve Bureau purchasing and storage of tungsten concentrate directly affects price. Since 2013, purchasing and storage for 5 times of tungsten concentrate, 3 of them have succeeded and affects tungsten raw material price.
9.Fanya Metal Exchange purchased a lot of APT since 2014, over 30000 tons of APT is purchased in 2014. But the price keeps sliding due to the increase of capacity. The stop of purchasing and returned of goods in the market in the beginning of 2015 causes tungsten price slumps in 2015. There is still 29651 tons of APT is stocked in Fanya, the potential effect can not be ignored.
10.Tungsten concentrate trade situation. When price difference is more than profit, capita flows into tungsten concentrate market. The trade operation will also affect market. When price of tungsten concentrate is less than manufacturing cost, traders purchase a lot as inventory then sell it at high price. The sliding of price in June,2016 is the classical operation method of traders.
Besides the above factors, environmental protecting policy, resource tax reform, weather condition in main factory, main stream big enterprises shut down, etc. are all the reasons that will affect price.
| AMT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Contained Ultra-Low Overpotential Oxygen Evolution Catalyst
- Details
- Category: Tungsten's News
- Published on Tuesday, 16 August 2016 16:21
- Written by chunyan
- Hits: 780
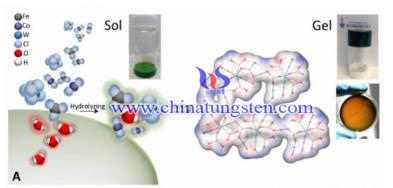 Hydrogen is an excellent energy carrier, and the way of solar-powered electrolysis of water to produce hydrogen is an effective method for energy storage. In the traditional SPEWE, since the high oxygen evolution potential, oxygen will cause serious dissolution to the carrier of carbon; in the oxygen evolution reaction process, dissolution and passivation of anode will occur and to shorten its live when electrode with high oxygen evolution overpotential working under the high potential. The presence of multiple high-energy intermediate state makes the oxygen evolution reaction one of the most difficult steps in the electrolyzed water. Thus, the preparation of ultra-low overpotential oxygen evolution catalyst will help to achieve efficient electrolysis of water.
Hydrogen is an excellent energy carrier, and the way of solar-powered electrolysis of water to produce hydrogen is an effective method for energy storage. In the traditional SPEWE, since the high oxygen evolution potential, oxygen will cause serious dissolution to the carrier of carbon; in the oxygen evolution reaction process, dissolution and passivation of anode will occur and to shorten its live when electrode with high oxygen evolution overpotential working under the high potential. The presence of multiple high-energy intermediate state makes the oxygen evolution reaction one of the most difficult steps in the electrolyzed water. Thus, the preparation of ultra-low overpotential oxygen evolution catalyst will help to achieve efficient electrolysis of water.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Mesoporous WO3 Nanofibers Disease Diagnosis
- Details
- Category: Tungsten's News
- Published on Friday, 12 August 2016 09:21
- Written by chunyan
- Hits: 721
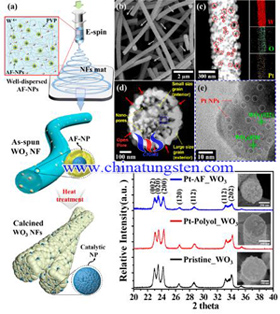 Recently, scientists have prepared mesoporous WO3 nanofibers, and discovered its new feature that this material has proved to have very large applications in reliability disease diagnosis. It is reported that the team took human exhaled gas as an object, and use of protein nano-cage module sensitization of electrospinning nanofibers (NFs) for detecting its tracking biological target sensitivity and selectively. In addition, the researchers prepared mesoporous WO3 nanofibers with Pt, Pd and Rh uniformly distributed; after the precious metal catalysis, tungsten trioxide gas sensors still has outstanding sensitivity even in conditions of wet environment, gas concentration level of one-billionth. The most exciting news is that this modified mesoporous WO3 nanofibers was confirmed to have a bright future in terms of reliability of disease diagnosis.
Recently, scientists have prepared mesoporous WO3 nanofibers, and discovered its new feature that this material has proved to have very large applications in reliability disease diagnosis. It is reported that the team took human exhaled gas as an object, and use of protein nano-cage module sensitization of electrospinning nanofibers (NFs) for detecting its tracking biological target sensitivity and selectively. In addition, the researchers prepared mesoporous WO3 nanofibers with Pt, Pd and Rh uniformly distributed; after the precious metal catalysis, tungsten trioxide gas sensors still has outstanding sensitivity even in conditions of wet environment, gas concentration level of one-billionth. The most exciting news is that this modified mesoporous WO3 nanofibers was confirmed to have a bright future in terms of reliability of disease diagnosis.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com