Synthesis of WO3 Thin Films with Ammonium Paratungstate for PEC Applications
- Details
- Category: Tungsten Information
- Published on Wednesday, 28 April 2021 03:16
Photoelectrochemical (PEC) water splitting is considered as one of the cleanest, most efficient, and sustainable routes for the production of hydrogen (H2) and an ideal solution for future energy demands. Metal oxide semiconductors are known to be suitable materials for PEC water splitting as they are inexpensive and very stable in aqueous solutions. Moreover, reaching high PEC water splitting efficiency via metal oxide semiconductors is not easy.
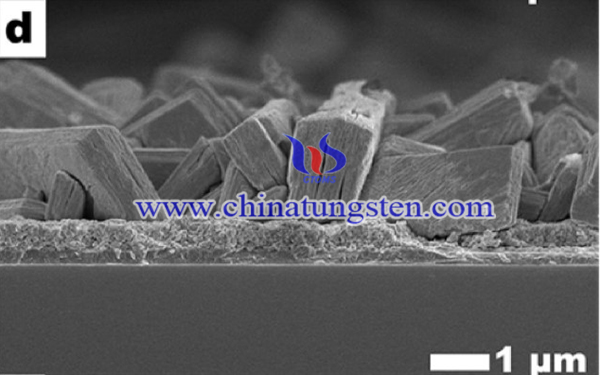
WO3 is regarded as a promising photoanode material for PEC. It is an excellent electron transporter (∼12 cm2 V−1 s−1), has a moderate hole diffusion length (∼150 nm), Unfortunately, the PEC activity of WO3 is limited due to its band gap, that allows the absorption of only 12% of the incident solar light . Hence, further improvements and modifications of WO3 are required for its efficient utilization as a photoanode in PEC water splitting systems.
Thus, porous WO3 thin films with particle size of 100 nm were obtained by a hydrothermal synthesis using ammonium paratungstate (APT) as material, the excellent photo activity is feasible for PEC applications. A significantly enhanced photocurrent of 3.33 mA cm−2 at 2 V vs had been observed.
The synthesis process of WO3 thin films is as follows:
1.0 g ammonium paratungstate (APT) was added to 94 mL de-ionized water and stirred to promote full dissolution. Two milliliters of concentrated H2SO4 (instead of HNO3) was added to the above solution and stirred for 1 h. To this, 4 mL of H2O2 was added and stirred for 1 h to form a clear peroxotungstic acid solution. This solution was then transferred to a Teflon-lined autoclave, filling it to 70%. A previously cleaned FTO substrate was placed inside the autoclave at an angle against the wall of the Teflon-liner with its conducting side facing down. Hydrothermal synthesis was then carried out at 150 °C for 4 h. After the synthesis, the autoclave was cooled to room temperature (299 K), and the resultant WO3-coated FTO was removed and washed with de-ionized water. The hydrothermally synthesized hydrated WO3 thin films were then converted to crystalline un-hydrated WO3 by annealing at 500 °C for 2 h (at a ramping rate of 3 °C/min) to form crystalline WO3; the formation of oxygen vacancies in WO3 was controlled by changing the annealing atmosphere (i.e., air, O2, and H2).
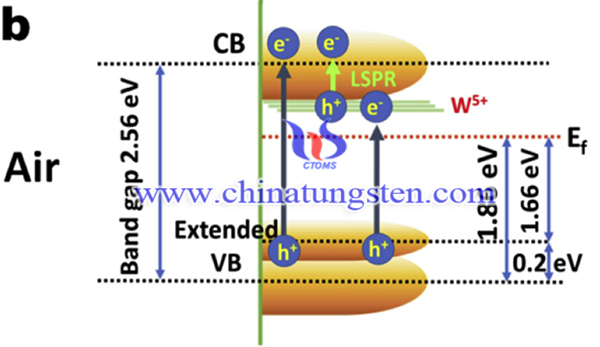
In conclusion, Porous WO3 thin films with particle size of 100 nm were obtained by a hydrothermal synthesis using APT as material. Annealing in air led to a critical phase transition and reconstructive transformation to yield a stable monoclinic phase with a significant amount of oxygen vacancies. the air annealed sample shows a maximum IPCE value of up to 2% in the wavelength range of 800–900 nm and ∼3% at nearly 1100 nm, the highest PEC activity obtained for the air annealed sample was attributed to an increase in the carrier density along with the narrowing of the band gap, which allows efficient light harvesting in the far visible and IR regions and charge transport during illumination.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com