Production Of Nanostructured Tungsten-Lanthanum Oxide Composites Using Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Friday, 13 November 2020 02:33
Tungsten is an important and prominent metal among the refractory metals due to an excellent combination of outstanding high temperature properties and high melting point (highest of all metals). Its high strength and corrosion resistance at elevated temperatures, good thermal conductivity and low thermal expansion makes it possible to use tungsten in high temperature applications. On the other hand, owing to its high hardness and wear resistance, high modulus of elasticity and compression strength, tungsten is an important constituent as an alloying element in tool steel, superalloys, stellites and hard metal industry.
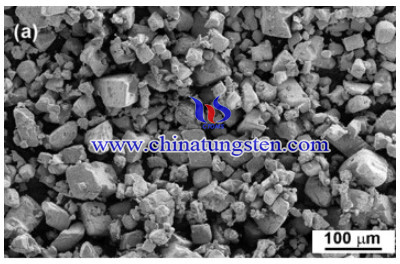
High-purity tungsten-lanthanum oxide composites (W-La2O3) nanopowders were produced by a wet chemical route. The precursor was prepared by the reaction of ammonium paratungstate (APT) with lanthanum salt in aqueous solutions.
The experimental procedures are as follows:
Lanthanum doped tungsten precursors with lanthanum content corresponding to W–La2O3, were synthesized from APT and lanthanum nitrate hydrate, La(NO3)3⋅xH2O. 50 g of as-received APT powder and 0.86 g of lanthanum nitrate hydrate were added in 153 mL of water. The reaction was carried out under stirring at room temperature. The solution was filtered after 24 h reaction (sufficient time to ensure complete reaction between APT and La ions) and the obtained powder was dried at room temperature. A sample from obtained dried powder was used for chemical analysis and was found to contain 69 wt.% W and 0.5 wt.% La. The precursor was calcined followed by reduction in a tube furnace. The calcination was carried out under nitrogen atmosphere at 450 °C for 1 h whereby the powder is transformed into oxide mixture. The reduction was done by heating under pure hydrogen atmosphere at 800 °C for 6 h. The heating rate up to the processing temperatures was 5 °C/min. In a separate experiment, the as-received APT was reduced to pure tungsten powder under the same conditions. In another set of experiments, the reduction was carried out in two steps; first at 600 °C for 2 h, then the temperature was raised to 800 °C and the reduction was allowed to proceed for 6 h. All samples were cooled overnight under a hydrogen flow.
The spark plasma sintering was carried out using Dr. Sinter 2050 SPS (Sumitomo Coal Mining Co., Japan) sinter reduced powders under vacuum using graphite dies. Powders were sintered at two different temperatures (1300 and 1400 °C) under 75 MPa pressure, for 3 min. Densities of sintered samples were measured. The relative densities (RD) were calculated using theoretical densities of tungsten and La2O3 as 19.25 g cm−3 and 6.57 g cm−3 respectively. Polished sintered samples were subjected to Vickers microhardness testing under 200 g load and a dwell time of 20 s at room temperature.
In conclusion, high purity nanocrystalline lanthanum oxide dispersed-tungsten powders were successfully synthesized by controlled chemical reactions from aqueous solution at room temperature for 24 h. The particles of the precursor powders were coated with La-containing products on the surface, rather than doped inside. This implies that distribution of oxide phase in bulk ODS metal is defined by APT particles size and its distribution in precursor powder. ODS–tungsten composites are successfully sintered by SPS technique at temperatures significantly lower than those in conventional sintering methods which avoided substantial grain growth. Tungsten matrix consists of micron sized grains having fine sub-grains with low angle boundaries. Hardness of sintered samples depends primarily on the degree of densification. Although, the higher sintering temperature (1400 °C) results in higher degree of densification and hence higher hardness, incipient melting of La–W–oxides occurs.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com