Phase Transformation in Tungsten Oxides Toward Energy Storage
- Details
- Category: Tungsten's News
- Published on Friday, 03 February 2023 21:42
It is quite important to understand the working mechanism of tungsten oxides from phase change to energy storage. Supercapacitors (SCs) and lithium-ion batteries (LIBs) are two typical types of efficient energy storage devices (ESDs), and electrochromic devices (ECDs) can also be considered as ESDs.
The operation of LIBs depends on the movement of Li+ ions between the cathode and the anode. Compared to pseudo-capacitors, the charging and discharging process of LIBs usually takes much longer time because redox reactions occur not only on the surface of the electrode but also in its deep volume.
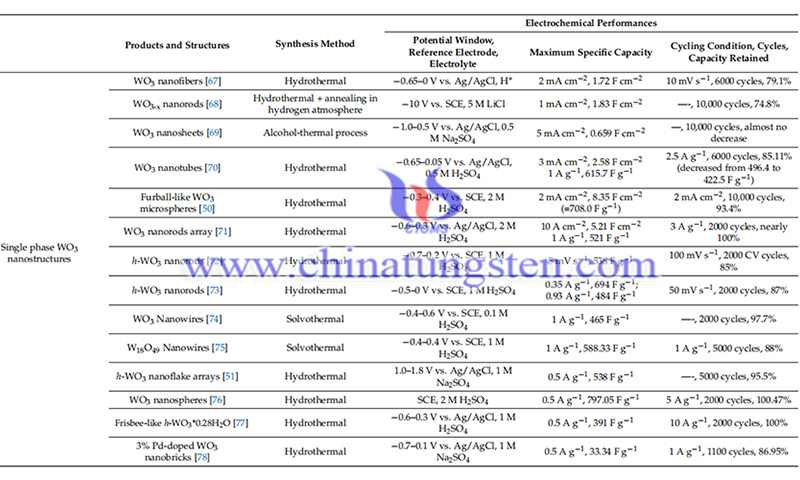
However, tungsten oxides cause structural collapse and rapid capacity drop during cycling. In addition, the low conductivity leads to poor rate performance. The morphology and phase transition of WO3 made by magnetron sputtering after initial complete discharge (at 0.01 V) and charging (at 4.0 V) were explored by scanning electron microscopy (SEM) and transition electron microscopy (TEM), revealing large volume changes of the phase transition.
WO3 is a cathodically conductive material. Its color changes from colorless to blue as a result of the insertion of ions and electrons when a negative voltage is applied. This process is reversible when the applied voltage changes to a positive value. Therefore, ECDs can be considered transparent ESDs. it has also been reported that these color changes occur due to band gap changes caused by ion insertion and extraction.
As mentioned above, the electrochemical reactions of WO3 in ECDs were found to be similar to those in SCs and LIBs, which is of great help in developing integrated devices based on WO3 for ESDs and ECDs. These three devices have the same sandwich structure. However, their different operating mechanisms require different requirements for tungsten oxide-based electrodes. For all three devices, a large specific surface area and good electrochemical conductivity of the tungsten oxide electrode are necessary to obtain fast Faradic reactions.
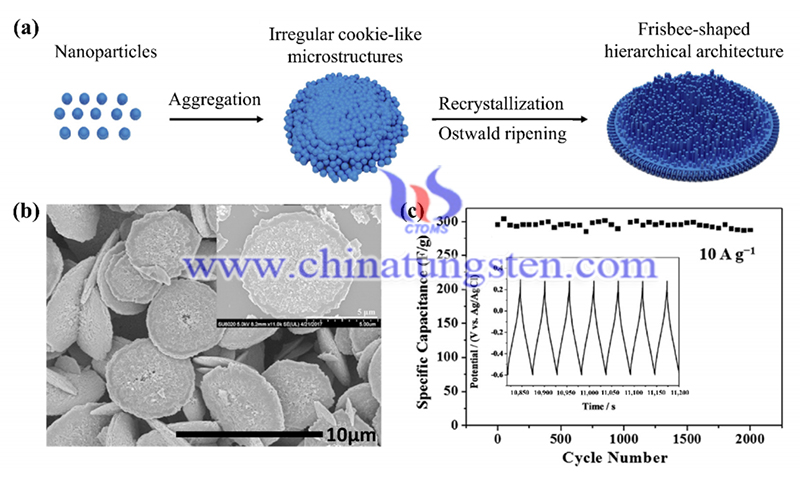
Reference: Han W, Shi Q, Hu R. Advances in electrochemical energy devices constructed with tungsten oxide-based nanomaterials[J]. Nanomaterials, 2021, 11(3): 692.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com