Crystal Structure of Tungsten Oxides
- Details
- Category: Tungsten's News
- Published on Friday, 03 February 2023 21:34
Tungsten trioxide (WO3) is the most widely used tungsten oxide today. Perfect tungsten oxides are ReO3-type cubic crystal structure materials in which octahedral WO6 are interconnected by parting the angles. In the WO6 octahedron, the W atom is located in the center and the remaining six O atoms form the octahedral framework. With changes in temperature and pressure, the WO6 octahedra tilt and rotate at certain angles, leading to the formation of several different phases: tetragonal, orthorhombic, monoclinic, triclinic, and cubic phases.
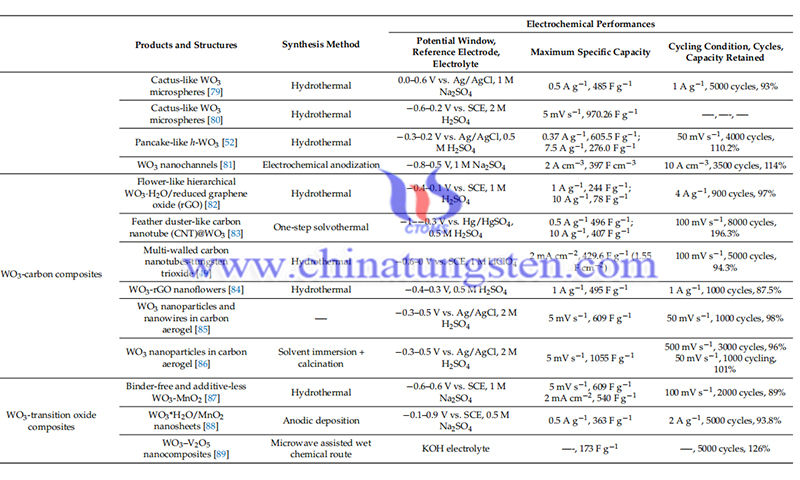
Inside WO3, there are some sites and tunnels between octahedra, so small diameter atoms such as H+, Li+ and K+ can be transferred into WO3 and stored. There is another phase of WO3, the hexagonal phase, which can be obtained from the hydration process of hydrated tungsten oxides. After the WO6 octahedral stacking, trigonal and hexagonal tunnels are formed along the c-axis. These tunnels facilitate the rapid transfer of ions and electrons, so the electrochemical activity of hexagonal tungsten oxides is better than that of other phases of WO3. The most common tungsten trioxide phases are the monoclinic phase (m-WO3), and the hexagonal phase (h-WO3).
Oxygen deficiency is very common in naturally occurring WO3, leading to the presence of sub-stoichiometric tungsten oxides, WO3-x (0 < x < 1), where the valence of W may be +3, +4, or +5. Of these, W18O49, W20O58 and W24O68 are the most common, and their internal oxygen deficiency promotes their electrical conductivity. This property makes WO3 an n-type semiconductor whose conductivity can be tuned by controlling the amount of O vacancies in it.
Tungsten oxides made by liquid-related methods are usually hydrated tungsten oxides before heat treatment, i.e., WO3-xH2O, which mainly include WO3-0.33H2O, WO3-0.5H2O, WO3-H2O, and WO3-2H2O. The crystal structure of WO3-xH2O is mainly determined by the value of x. For example, the structure of WO3-H2O is that the H2O is located in the interlayer gap of the WO6 octahedron, while the structure of WO3-2H2O is that in addition to the similar H2O molecules in WO3-H2O, another class of H2O molecules is directly connected to the tungsten atoms at the bottom or top of the octahedron.
This structure facilitates the easy transport of ions and electrons, especially protons, through their internal hydrogen bonding network. Typically, hydrated tungsten oxide has better electrical conductivity than pure tungsten oxide, translating into enhanced electrochemical properties.
Reference: Han W, Shi Q, Hu R. Advances in electrochemical energy devices constructed with tungsten oxide-based nanomaterials[J]. Nanomaterials, 2021, 11(3): 692.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com