Protein Process May Revolutionize Rare Earth Extraction
- Details
- Category: Tungsten's News
- Published on Monday, 01 November 2021 21:48
Protein process may revolutionize the rare earth extraction. A team led by Joseph A. Cotruvo Jr. of Pennsylvania State University and Dan M. Park of Lawrence Livermore National Laboratory has now developed an alternative process to mine these resources without the need for organic solvents, improving access to rare earth elements (REEs) while helping to avoid industrial waste, according to Chemical & Engineering News.
According to the team, a protein bound to REEs could be used to extract and separate these valuable metals from low-grade sources.
Rare earth elements include 15 lanthanides as well as scandium and yttrium, many of which are in high demand for products such as electric vehicles, wind turbines and light-emitting diodes.
The protein process relies on lanmodulin, a small acid-resistant protein with a strong affinity for lanthanides. It is produced by certain bacteria that digest methane.
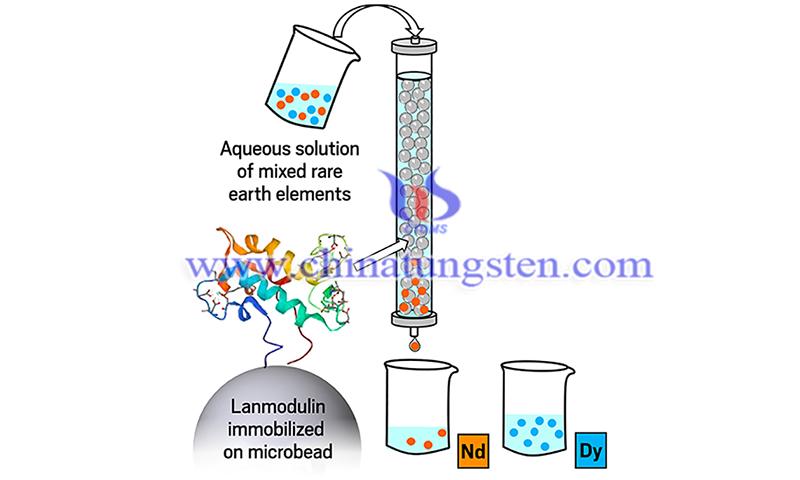
When an acidic solution of metal ions passes through the column, lanmodulin takes REEs from the solution while allowing other elements such as copper or zinc to pass right through, according to the report.
The REEs can then be released by changing the pH of the solution or by adding counter ions such as citrate to chelate the REEs, Cotruvo said, adding that the acid and the column itself can be reused many times during this process.
The team tested the purification column on a solution leached from coal ash. A single pass through the column was sufficient to produce an enriched solution in which 88% of the metal ions were REEs, a 2,000-fold increase in purity.
"It's impressive because it starts with less than 0.1 percent REEs, so it's a really big upgrade," Cotruvo said. By carefully adjusting the pH, the researchers were able to help rare earth extraction from the column in two batches, containing lighter and heavier REEs.
The protein process can also completely separate mixtures of two REEs, including neodymium and dysprosium, which are typically found together in e-waste containing rare earth magnets, the report said.
Cotruvo said, "This is one of the most important separations if we are to recover e-waste. Starting with a solution that mimics the composition of this type of waste, the researchers used several separation cycles to recover more than 80 percent of each element, each with a purity of more than 99 percent."
Lena J. Daumann, a bioinorganic chemist at Ludwig Maximilian University Munich, said, "This is a good demonstration of how a natural system can be used to solve the problem of separating lanthanides with little change. This protein-based approach, once scaled up and optimized as a continuous process, could be a really promising technique for low-level sources."
REEs are the 'vitamins of chemistry’, says Daniel Cordier, a rare earth mineral commodity specialist with the U.S. Geological Survey: "REEs help everything perform better, and they have their own unique properties, especially in terms of magnetism, temperature resistance and corrosion resistance."
- Rare Earth Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com