High-Efficiency Hydrogen Production Using Pt/α-MoC Catalyst
- Details
- Category: Tungsten's News
- Published on Saturday, 06 February 2021 23:20
Pt/α-MoC catalyst realizes high-efficiency catalytic hydrogen (H2) production process. Platinum (Pt) atomically dispersed on α-molybdenum carbide (α-MoC) enables low-temperature, base-free hydrogen production. Polymer electrolyte membrane fuel cells (PEMFCs) running on hydrogen are attractive alternative power supplies for a range of applications, with in situ release of the required hydrogen from a stable liquid offering one way of ensuring its safe storage and transportation before use.
The use of methanol is particularly interesting in this regard, because it is inexpensive and can reform itself with water to release hydrogen with a high gravimetric density of 18.8 per cent by weight. But traditional reforming of methanol steam operates at relatively high temperatures (200–350 degrees Celsius).
Pt/α-MoC catalyst realizes high-efficiency, low-temperature catalytic hydrogen production process. As a typical representative of clean energy, hydrogen energy is highly regarded, but its chemical properties are lively, and the efficiency and safety issues in the process of preparation, storage and transportation have been plagued by industry and research circles.
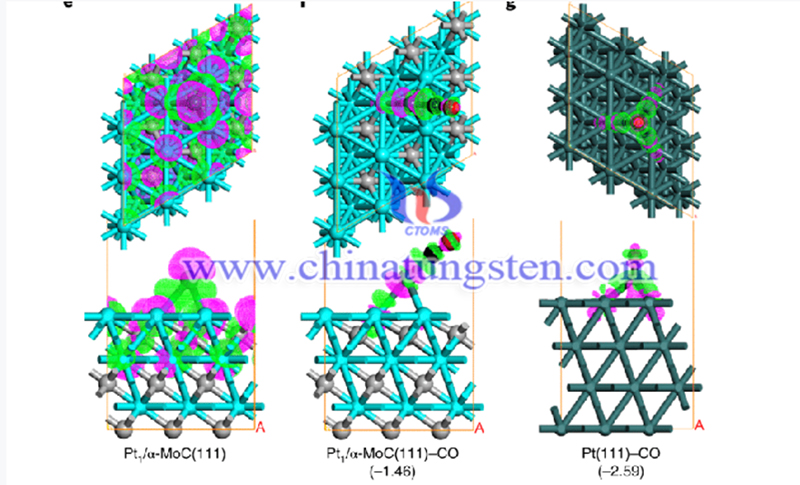
To find the best solution for hydrogen energy production, storage and transportation, researchers of the School of Chemistry and Molecular Engineering of Peking University have worked tirelessly for several years. Recently, the research team has made new progress in joint research. The relevant results have been published in Nature.
The water-gas shift (WGS) reaction is an industrially important source of pure hydrogen at the expense of carbon monoxide and water1,2. This reaction is of interest for fuel-cell applications, but requires WGS catalysts that are durable and highly active at low temperatures3. It is critical to crowd the α-MoC surface with Pt1 and Ptn species, which prevents oxidation of the support that would cause catalyst deactivation. The design of highly active and stable catalysts for effective activation of important molecules such as water and carbon monoxide for energy production.
The research team designed and synthesized an interface catalytic structure composed of high-density, high-dispersion atomic-grade platinum (Pt) species and cubic molybdenum carbide (α-MoC) to prepare a highly efficient and stable Pt/α-MoC catalyst. The catalyst can be used to catalyze the water-gas shift (WGS) hydrogen production reaction, which is also the WGS catalyst with the best catalytic performance reported so far.
WGS reaction is one of the important ways to produce pure hydrogen (H2) in the field of energy and chemical industry. Limited by the chemical equilibrium, the WGS reaction is more favorable at low temperatures, which puts forward higher requirements on the activity and durability of the catalyst.
"α-MoC has excellent properties of dissociating water molecules (H2O). The dissociation of H2O can be achieved at room temperature to release hydrogen. However, if the hydroxyl species adsorbed and dissociated on the surface cannot be converted in time at a certain temperature, it will be in the long-term catalytic process. There is the problem of deep oxidation of α-MoC which leads to catalyst deactivation. Therefore, how to achieve the perfect match of H2O and carbon monoxide (CO) adsorption activation rate is a key scientific issue to further improve catalyst reaction activity and long-term stability." The researcher told the China Science Daily.
At present, the technology for preparing Pt/α-MoC catalyst in hydrogen production has been patented. The researcher said that by studying the mechanism of the Pt/α-MoC catalyst, the research team also revealed the reasons for the high efficiency of the catalyst, providing new ideas for the development of subsequent high-efficiency catalysts.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |