Quantum Diffusion of Heavy Defects Like Tungsten Defies Arrhenius' Law
- Details
- Category: Tungsten's News
- Published on Monday, 23 March 2020 19:21
Defect clusters containing around 100 tungsten atoms (atomic mass 184) have been experimented by researchers from Japan, France, and the United Kingdom, and the test has shown out that at cryogenic temperatures, "massively heavy" atoms can mechanically move quantum within a crystalline material which defies the Arrhenius’ law.
This conclusion contradicts the generally held notion that only hydrogen or helium atoms are light enough to migrate through materials in this way. This is beneficial to our research on the low-temperature dynamics of defects and could lead to new applications in materials science and engineering.
A perfect crystal is a purely theoretical concept. However, crystals in the real world usually contain defects, which severely degrade the mechanical properties of the material. Therefore, understanding how these defects diffuse and interact is important for a wide range of processes in materials science and metallurgy, including alloying, precipitation, and phase transitions.
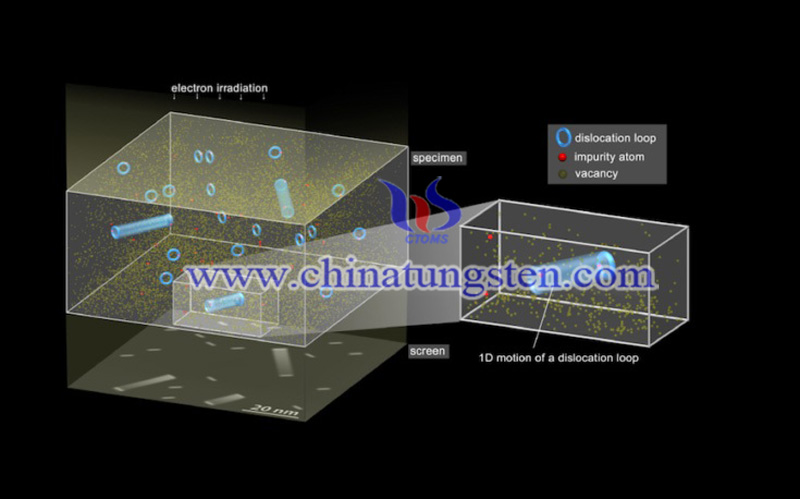
Defects are bound to so-called static trapping centers (usually impurity atoms in the crystal), so they need to "de-trap" before they can travel. For elements heavier than hydrogen or helium, de-trapping is thought to occur by thermal activation, and defect diffusion rates typically obey Arrhenius' law, which is a century-empirical rule, describing how the rate of chemical reactions varies with temperature. When the material is in an extremely low-temperature environment, Arrhenius's law states that the transmission of heavy atom defects will be greatly slowed down and may even become "frozen". It can be used to model the temperature variation of diffusion coefficients, the population of crystal vacancies, creep rates, and many other thermally-induced processes or reactions.
A research group teamed by Shimane University, Nippon Steel, Nagoya University and Osaka University in Japan, the CEA and CNRS in France, and the University of Leeds and Culham Centre for Fusion Energy in the UK have discovered a type of flaws contradicting this law. This defect occurs when excess atoms of the same type that make up the material's crystal lattice are misplaced in a regular stack. These "self-interstitial atoms" (SIAs) cause deformation and stress in the lattice structure, and the researchers have studied how defect clusters pass through tungsten samples at low temperatures.
The research team irradiated tungsten with a 105K high-energy (2000kev) electron beam to create self-interstitial atomic defects and vacancies, that is, lattice sites with "missing" atoms, which is the counterpart of self-interstitial atoms. When the sample is at 300K, self-interstitial clusters can be nucleated, grow to nanometer size and bind to the capturing center. Then, the sample was aged at 300 K to nucleate self-interstitial clusters, grow to nanometer size, and bind to the capturing center.
At these temperatures, the researchers noticed that the defects were thermally immobile and remain dispersed throughout the sample. Their next step was to illuminate the sample with lower energy (100-1000kev) electron beam. The energy of the second beam is too low to generate additional SIA, but high enough to move the vacancies in a non-thermal state and cause the trapped self-interstitial clusters to "de-trapped". This de-trapping can be achieved through thermal and quantum mechanical mechanisms.
The researchers said that by measuring the movement frequency of these clusters using an in-situ transmission electron microscope, they could distinguish between purely thermal motion and motion caused by quantum mechanical processes. Surprisingly, they found that quantum-assisted defect de-trapping caused the low-temperature diffusion rate to be several orders of magnitude higher than Arrhenius' law.
The lead author of the study, Kazuto Arakawa, said: "Our results show that quantum transport, even of heavy defects, becomes dominant below around one-third of the Debye temperature (which is the approximate temperature below which quantum effects may be observed)."
Arakawa said the new discovery will impact a variety of fields, including materials science and engineering, because any low-temperature processes associated with defect transmission or diffusion are important. The term "low temperature" is relative: beryllium, for example, has a Debye temperature of 1280 K, so even the diffusion of beryllium defects may be a major phenomenon even at room temperature.
He added that the quantum diffusion of heavy defects such as tungsten defies Arrhenius’ law could have even more far-reaching consequences. So far, most observations of atomic move in crystals at low temperatures were interpreted using the law. The fact that heavy defects move faster than expected at these low temperatures suggests that the materials-science community may need to revisit and reinterpret previous low-temperature experiments.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com