Molybdenum-Catalyzed Ammonia Production Subverts Haber-Bosch Process
- Details
- Category: Tungsten's News
- Published on Monday, 21 October 2019 15:17
Molybdenum-catalyzed ammonia production has been developed by researchers at the University of Tokyo. This new ammonia-making process is light, efficient, clean, low-energy, and low-cost based on a special Samarium-Water Ammonia Production (SWAP) promises to scale down ammonia production and improve access to ammonia fertilizer to farmers everywhere which subverts the Haber-Bosch process. Related research results are published in Nature.
The production of ammonia from nitrogen gas is one of the most important industrial processes, owing to the use of ammonia as a raw material for nitrogen fertilizers. Currently, the main method of ammonia production is the Haber-Bosch process, which operates under very high temperatures and pressures and is therefore very energy-intensive. Which is regarded as one of the greatest inventions of the 20th century and promotes the development of subversive technology. However, this method has the drawback of high energy consumption.
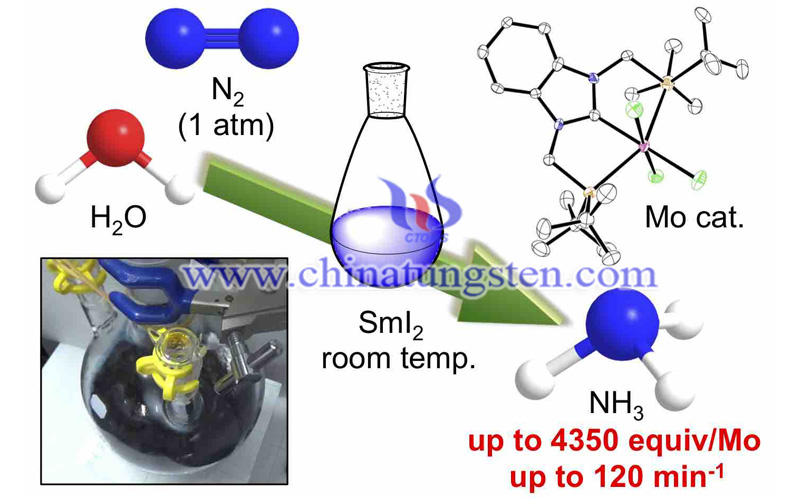
The traditional process converts 10 percent of its source material per cycle only, so it needs to be run multiple times to fully exhaust. One of these source materials is hydrogen produced using fossil fuels. This is chemically combined with nitrogen at temperatures of about 400-600 degrees Celsius and pressures of about 100-200 atmospheres, also at a great energy cost. Professor Yoshiaki Nishibayashi and his team from the University of Tokyo's Department of Systems Innovation hope to improve the situation with their SWAP process.
The SWAP process improves this situation. Yoshiaki Nishibayashi explained that "Worldwide, the Haber-Bosch process consumes 3 to 5 percent of all-natural gas produced, around 1 or 2 percent of the world's entire energy supply. In contrast, leguminous plants have symbiotic nitrogen-fixing bacteria that produce ammonia at atmospheric temperatures and pressures. We isolated this mechanism and reverse engineered its functional component - nitrogenase."
Over the years, Nishibayashi and his team used laboratory-made catalysts to try to replicate the functional performance of nitrogenase. Other researchers tried but their catalysts only produce dozens to several hundred ammonia molecules before they expire. Nishibayashi's special molybdenum-based catalyst produces 4,350 ammonia molecules in about four hours before it expires.
According to Nishibayashi, the speed of ammonia production by the SWAP process at 90 percent efficiency. The benefits of this process are obvious given the gargantuan energy savings in the process and sourcing of raw materials. Besides, anyone with the proper source materials can perform SWAP on a table-top chemistry lab, while the traditional process requires large-scale industrial equipment. This new process offers opportunities for companies that lack the investment capacity of expensive equipment.
SWAP produces nitrogen from the air similar to the Haber-Bosch, but the special molybdenum-catalyzed ammonia production combines this with protons from water and electrons from the samarium. Samarium also is known as Kagan’s reagent, is currently mined and is used up in the SWAP process. However, samarium can be recycled with electricity to replenish its lost electrons and researchers aim to use cheap renewable sources for this.
Nishibayashi and his team found something as common as water could serve as the proton source. Their molybdenum catalyst is special. This is the first artificial nitrogen-fixing reaction to reach a rate close to that we see nitrogenase produce in nature. The new process subverts the Haber-Bosch process. The molybdenum-catalyzed ammonia production presents an opportunity for further research in the field of catalytic nitrogen fixation.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com