Iron-Doped Tungsten Oxide Nanoreactor Provides New Ideas for Nitrogen Fixation
- Details
- Category: Tungsten's News
- Published on Thursday, 27 February 2020 20:38
The iron-doped tungsten oxide (W18O49) nanoreactor provides a new idea for electrocatalytic efficient nitrogen fixation. Recently, a team of researchers from Jian Liu of the Institute of Chemicals of the Chinese Academy of Sciences and the team of Ji Liang from the University of Wollongong, Australia, developed an iron-doped W18O49 nanoreactor through the strategy of defect-doped iron doping which achieved higher ammonia yield and higher Faraday efficiency at low potential, providing new ideas for efficient electrocatalytic nitrogen fixation.
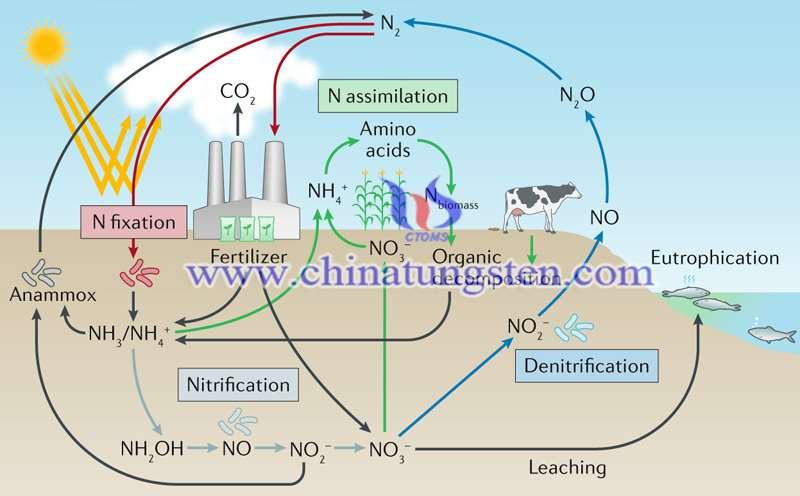
Ammonia is one of the important inorganic chemical products, which provides raw materials for the production of urea and fertilizers. The traditional ammonia synthesis uses the Hubble-Bosch method, which triggers high energy consumption, high pollution, and requires high temperature-pressure conditions. The electrocatalytic ammonia synthesis can be carried out at room temperature and atmospheric pressure, which owns the advantages of high energy efficiency and low emissions.
In the process of electrocatalytic ammonia synthesis, there is an issue that it is difficult to activate nitrogen. Besides, at the same time as the electrochemical synthesis of ammonia at room temperature, there is a competitive reaction, that is, a hydrogen evolution reaction. Due to similar energy barriers, it is difficult to achieve high Faraday efficiency and ammonia yield.
The research team controlled the oxygen vacancy state in tungsten oxide through the iron doping strategy of nano-reactor defect engineering. Via it combined with Ab initio calculations, it was found that by utilizing the inherent low hydrogen binding energy of tungsten oxide, the maximum active site (tungsten) exposure was obtained through defect engineering regulation, which greatly weakened the hydrogen evolution reaction and reduced the electrochemical nitrogen reduction at room temperature.
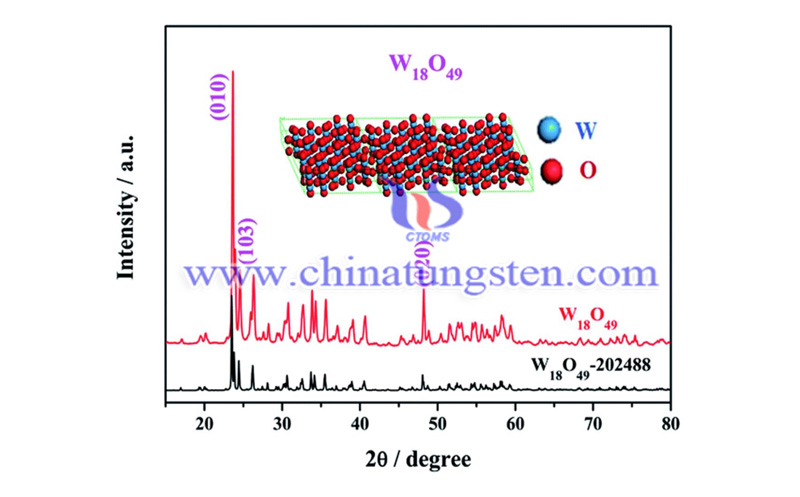
Therefore, at a very low potential of -0.15 volts relative to the reversible hydrogen electrode, the amount of tungsten oxide with optimized iron content was optimized, and a higher ammonia yield (24.7 μg / (hour * mg catalyst)) and a higher Faraday efficiency were obtained. (20.0%). It verified that the iron doping strategy and the adjusted oxygen vacancy state can jointly optimize the electronic state and surface structure of W18O49, thereby improving the adsorption strength of nitrogen and obtaining a low-potential reaction path. This work provides new ideas for the design and regulation of catalysts for various electrochemical reaction processes.
The iron-doped tungsten oxide nanoreactor could enable low-potential electrochemical nitrogen fixation. The above work was recently published in the journal Angew. Chem. Int. Ed.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com