Atomic Structure of Tungsten Disulfide
- Details
- Category: Tungsten Information
- Published on Sunday, 11 September 2022 22:22
The atomic structure of tungsten disulfide (WS2) consists of a stack of three layers formed by a transition metal layer (W atom) sandwiched between two S-atom layers, each with a hexagonal lattice structure. In the three-layer stack, W and S atoms are bonded together by strong ion-covalent bonds. WS2 formed by these three layers is held together by weak van der Waals interactions, which allow mechanical exfoliation of the WS2 layer. In the bulk phase, polymorphism is a unique feature of TMDs.
Linus Pauling first determined the structure of transition metal dichalcogenides (TMDs) in 1923, and since 1960 more than 60 TMDs have been discovered. 2004 saw the isolation of stable graphene from graphite, which opened a new path for the application of ultrathin films of TMDs. There are 44 TMD compounds that can form stable two-dimensional (2D) structures and they are composed of MX2, where M is a transition metal and X is S, Se, or Te.
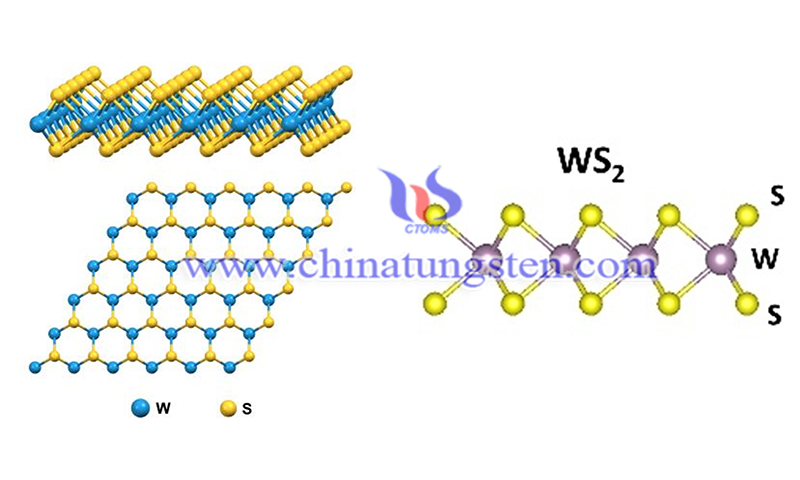
These compounds exhibit the physical properties of metals, semiconductors, superconductors, and insulators. Among these TMDs, WS2 has a unique band form, which stems from its semiconductor-related properties such as broadband spectral response properties, ultra-fast bleaching recovery, and excellent saturable light absorption.
The atomic structure of WS2 depends on the filling of d orbitals in the transition metal. tungsten disulfide has three possible stacking structures, namely 1T, 2H, and 3R. 1T is formed by tetragonal symmetry and octahedral coordination. n the 2H and 3R phases, the d orbitals of the transition metals are divided into three energy levels: dz2, dx2-y2, xy, and dxy, yz.
For monolayer WS2 films, the 2H is the most stable and common one. In addition to the more common 1T, 2H, and 3R stacking structures mentioned in this paper, WS2 also has a 1T’ form, which has attracted a great deal of attention due to its excellent topological insulating properties.
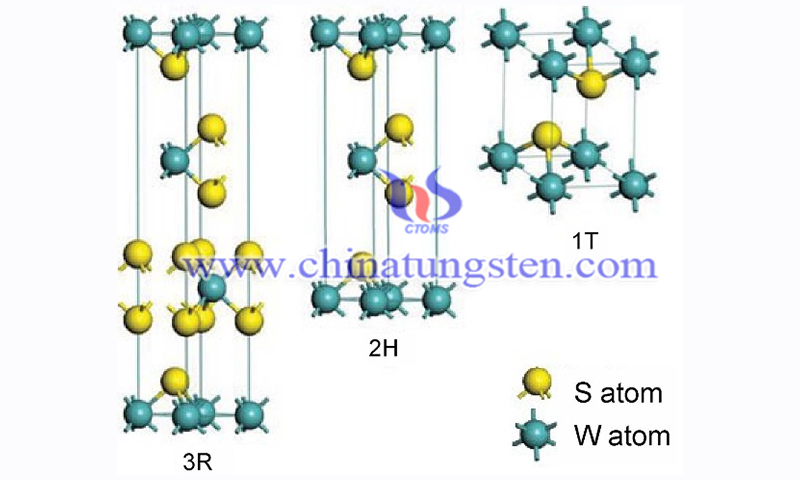
To change the atomic structure of WS2, lithium-ion intercalation and non-intercalation phase change methods are often used. Lithium-ion intercalation is a reversible process. When an excess charge is added, the 2H phase transition becomes unstable and changes to the 1T phase; however, when the lithium-ion is removed from WS2, the excess charge is removed. Subsequently, tungsten disulfide, which is in the 1T phase, reverts to the most stable 2H phase.
The intercalation-free phase change method consists of stimulation by infrared laser and electron beam irradiation. These reactions are irreversible. These reactions produce irreversible brass vacancies, which are the driving force for the transition from 2H to 1T. However, this method is destructive and cannot reverse the phase transition from 2H to 1T. These phase transitions can change the properties of WS2 to those between a semiconductor and a metal.
Cited Article: Ding J, Feng A, Li X, et al. Properties, preparation, and application of tungsten disulfide: A review [J]. Journal of Physics D: Applied Physics, 2021, 54(17): 173002.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com