Photocatalysis of Tungsten Disulfide
- Details
- Category: Tungsten Information
- Published on Wednesday, 31 August 2022 21:54
Tungsten disulfide (WS2) is a semiconductor with a band gap, which gives WS2 a wide range of light absorption, and therefore, WS2 can be considered a promising photocatalyst for photocatalysis degradation of organic pollutants and hydrogen production from water decomposition. WS2 extends the light absorption region to the long-wave direction, and through morphological tuning, WS2 can achieve near-infrared photocatalytic activity.
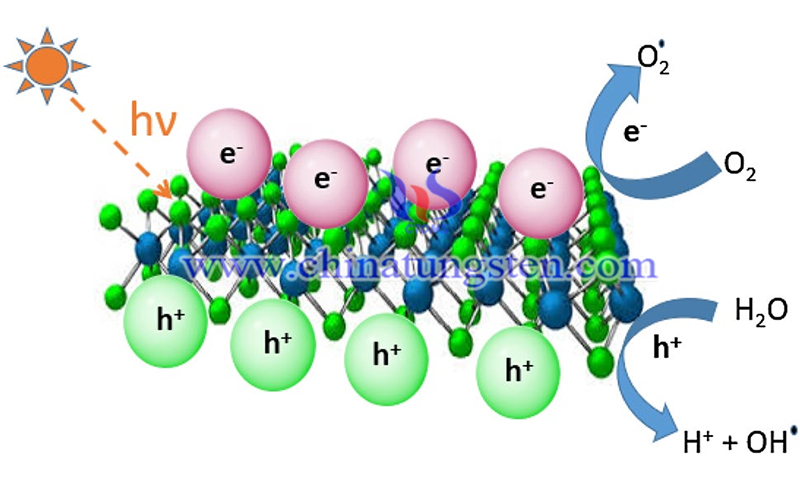
The use of semiconductor catalysts to degrade pollutants and catalyze the decomposition of water to produce hydrogen under light illumination is considered to be the most effective method to solve environmental and energy problems at this stage. In general, a highly efficient semiconductor catalyst must meet at least three conditions. First, the catalyst must have a suitable band gap and band sites; second, the photogenerated electrons and holes in the catalyst should be separated effectively and transferred rapidly to avoid electron-hole recombination; third, the surface of the catalyst should have suitable reaction sites to facilitate the chemical reaction.
(Picture source: Ashraf et al./ Applied Nanoscience)
For example, the photocatalytic activity of tungsten disulfide under near-infrared irradiation was first investigated by Sang et al. It was found that WS2 nanosheets possess high photocatalytic activity in near-infrared light. WS2 nanosheets show strong photocatalytic activity in ultraviolet and visible light, indicating that it is a special and promising broad solar spectrum photocatalyst. In addition, the large surface area is beneficial to improving the photocatalytic activity of WS2.
The prepared WS2 (P-WS2) showed higher photocatalytic activity than commercial bulk WS2 (C-WS2) and MOS2 (C-MoS2) in the degradation of rhodamine B (RhB) dye under visible light irradiation. However, a drawback of WS2 is that strong recombination between photogenerated charge carriers remains a challenge. An effective separation strategy is thought to employ photocatalysts composed of two different semiconductor phases to form a heterogeneous structure, which can lead to a vectorial transfer of electrons and holes from one semiconductor to the other.
Recently, researchers prepared polycrystalline hybrid WO3/WS2 systems that showed higher organic pollutant degradation efficiency than WS2 or WO3. In addition, compounding with carbon is a promising approach to improve their electro-transport performance. For example, Prabhakar Vattikuti et al. synthesized carbon-encapsulated exfoliated WS2 nanosheets with core-shell nanostructures that exhibited high photocatalytic activity. This facile nanostructure can form a distinct cross-linked heterostructure between the carbon shell and the exfoliated WS2, facilitating the separation of photogenerated electron and hole pairs.
In addition, the highly porous carbon shell on exfoliated tungsten disulfide promotes ion movement in the electrolyte and gives mechanical strain by adequate shielding during charging and discharging. EIS analysis confirms that the carbon material on WS2 increases its charge transfer and surface area. By doping with other elements, the electronic band structure can be adjusted to improve the absorption of sunlight. Therefore, combining with doped materials is also a feasible approach.

(Picture source: Ashraf et al./ Applied Nanoscience)
As mentioned earlier, WS2 is not suitable as photocatalysis alone for overall water separation. However, WS2 has been widely reported as a synergistic catalyst for photocatalytic water separation in combination with other semiconductors. For example, Mahler et al. prepared a TiO2-WS2 nanocomposite and found that the use of twisted 1T-WS2 nanostructures possessed a higher H2 evolution rate than the use of the conventional 2H-WS2 form.
The rate of hydrogen production from TiO2 dispersions in a mixture of water and methanol under the irradiation of 300 W Xe light source is 700 μmol-g-1.h-1. Adding the synthesized 1T-WS2 nanosheets, this rate can be increased to 2570 μmol-g-1.h -1, which is more than three times higher than that of pristine TiO2. In contrast, the 2H-WS2 nanostructure leads to a decrease in the hydrogen evolution rate to 225 μmol-g-1.h-1, which is due to the fact that the active sites of 1T-WS2 are more exposed than those of 2H-WS2.
Finally, 3R-WS2 is the least studied polycrystal because of its nearly insulating properties. Zong et al. prepared a WS2/CdS composite used as photocatalysts and the rate of H2 evolution of CdS under visible light irradiation is prominently improved by applying WS2 as a co-catalyst. At the optimal loading (420 μmol-g-1.h-1), the H2 evolution rate on WS2/CdS was 28 times higher than that on CdS alone.
In addition, Xiang et al. investigated layered CdS/WS2/graphene [C(WG)x] nanorods and explored the most suitable content of layered WS2-graphene (WG) in ternary composites at 4.2 wt% [C(WG)4.2], exhibiting visible light photocatalytic H2 production rate of 1842 μmol-g-1.h -1. Therefore, WS2 nanomaterials are potentially promising for photocatalytic applications.
Article Source: Sun, CB., Zhong, YW., Fu, WJ. et al. Tungsten disulfide nanomaterials for energy conversion and storage. Tungsten 2, 109–133 (2020).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com