Red Phosphors Fabricated via Ammonium Paratungstate by Sol-Gel Method
- Details
- Category: Tungsten Information
- Published on Tuesday, 06 April 2021 17:19
White light-emitting diodes (LEDs) are considered as next generation lighting devices and gained promising attention because of their attractive advantages such as small size, long reliability, low energy consumption, high luminescent efficiency and eco-friendly nature over the conventional incandescent and fluorescent lamps. A technique which combines UV LED and tri-color (Red, Green, Blue) phosphors is a solution to achieve a better display. The combination produces bright white light with high color rendering index and low correlated color temperature. unfortunately, the performance of red phosphor is much weaker than the performance of blue and green phosphors.
Rare earth elements, Eu3+ ions (Europium) are highly used as the luminescence activator in the red-emitting phosphors owing to their efficient f-f transitions. Recently, the investigations on the mixed tungsto-molybdate have become more interesting host materials for doping Eu3+, owing to their low phonon energy, good thermo-chemical stability, strong absorption in the near UV and blue wavelength region, effective energy transfer from luminescent host to activator and efficient red emission under the near UV excitation. A Eu3+ activated tungsto-molybdate red phosphors with excellent color purity has been conducted using ammonium paratungstate(APT) as tungsten source.
The synthesis process of Eu3+ (y = 0.0–1.0) activated LiGd1-y(WO·5Mo 0·5 O4)2 red phosphors were as below:
Eu3+ (y = 0.0–1.0) activated tungsto-molybdate red phosphors were synthesized via a polymeric complex sol gel method. Lithium nitrate (Analytical grade, SRL), gadolinium nitrate (99.9%, Alfa Aesar), europium nitrate (99.9%, Alfa Aesar), ammonium paratungstate (APT) (99%, CDH), ammonium molybdate tetrahydrate (AMT) (99%, SRL), ethylene glycol (Analytical grade, SRL) and citric acid (Analytical grade, SRL) were directly used as precursors without further purification. In this typical process, the deionized water was utilized as solvent.
Metal nitrates, LiNO3, Gd(NO3)3 and Eu(NO3)3, APT and AMT were dissolved in deionized water as separate solutions. Citric acid as a chelating agent was added to chelate the metal ions by its carboxylic groups in the nitrates and tungsto-molybdate metallic solution. The pH of the total solution was adjusted to 5.5 through the addition of ammonia solution. Ethylene glycol as a binding agent was mixed with the final metal-citrate solution to proceed with the esterification reaction and to form a polymeric-citrate complex solution. This solution was continuously stirred and heated at 80 °C for 5 h in a silicone oil bath (uniform heating) with a resistive coil heater to obtain the semi-transparent gel. Further, the polymer gel was prefired at 250 °C for 30 min and calcined at a temperature of 800 °C for 4 h using resistive muffle furnace to obtain the Eu3+ activated tungsto-molybdate phosphors.
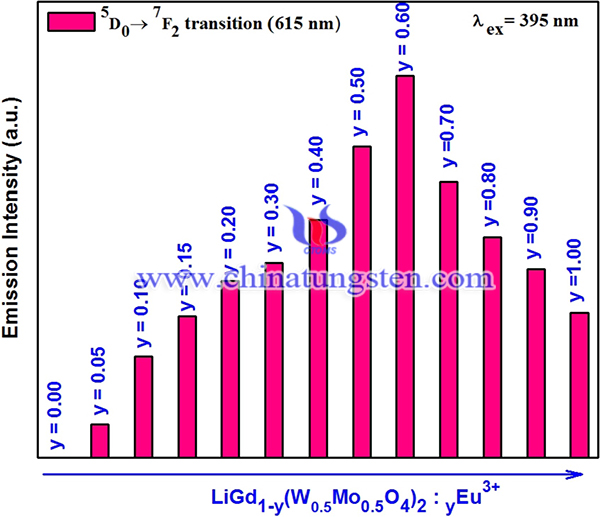
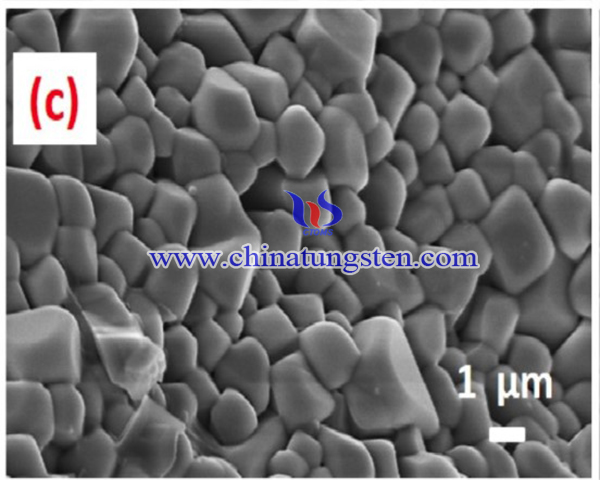
In summary, a series of Eu3+ activated LiGd(W0.5Mo0.5O4)2 red-emitting phosphors were successfully synthesized via polymeric complex sol-gel method. Surface micrographs of phosphors indicate the bulged irregular spherical morphology for the particles with the size range of 1–3 μm. These polyhedron microcrystals appears smooth and flatten surface with sub-angular edges. This helps to pack these particles with low number of pores and high packing densities, so that the phosphors are suitable for the fabrication of UV LED based applications. The intensity of red emission (5D0 → 7F2 transition) at 615 nm of the phosphors increases in the beginning of the concentration of Eu3+ ions and attains maximum for y = 0.60. Further increasing the Eu3+ concentration, the intensity of red emission decreases because of the concentration quenching behavior. that the lifetime of Eu3+activated tungsto-molybdate phosphors were reduced from 1.299 to 0.906 ms with the concentration of Eu3+ ions increases from 0.05 to 1.0 and the reason for this is the occurrences of self-generated quenching between Eu3+ ions in the samples. Color purity of the phosphors are estimated to be 91.77% and the results indicates that Eu3+ (y = 0.60) activated LiGd(W0·5Mo0·5O4)2 phosphors possess high color. Therefore, this tungsto-molybdate phosphors can be a potential candidate for red emission source in the production of UV based (excited) white LED applications.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com