Tungstic Manganese Calcium / Mesoporous Tungsten Trioxide Composite Catalyst
- Details
- Category: Tungsten Information
- Published on Sunday, 19 August 2018 22:52
Hydrogen is a kind of clean energy with high calorific value, friendly environment and convenient transportation. The combustion product is water, which does not cause any harm to the environment. The earth is rich in water, easy to get and cheap. Sunlight is an ideal way to convert solar energy into chemical energy.
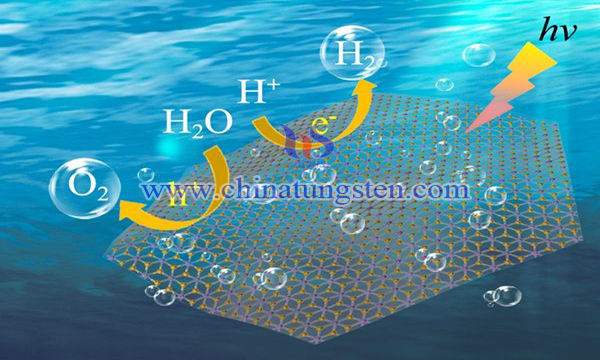
The reaction of photocatalytic decomposition of water with industrial and practical value is carried out in a photoelectrochemical cell. The decomposition of water by photochemical cells includes two half reactions: the reduction of photocathode water and the oxidation of photocathode water. The process of water oxidation on the photoanode is a four-electron reaction, which restricts the whole reaction. Therefore, it is particularly important to obtain photocatalytic anodes that can effectively realize photocatalytic oxidation of water to produce oxygen.
Researchers have developed a kind of tungsten-manganese-calcium/mesoporous tungsten trioxide composite catalyst which can be used to prepare photoanode, including active component I, Mesoporous Tungsten trioxide, active component II, layered manganese-calcium oxide, the composite catalyst can give full play to the catalytic advantages and obtain high yield products.
(1)Preparation of mesoporous tungsten trioxide. Phosphotungstic acid and mesoporous silica (KIT-6) with pore size of 7-8 nm were added to anhydrous ethanol under magnetic stirring. After stirring for 1-3 hours, the solid was centrifuged and dried. The solid was calcined at 400-600 ℃ for 3-6 hours and cooled naturally to room temperature. The obtained solids were then dispersed into hydrofluoric acid (1~5 mol/L), stirring at room temperature for 6 h and centrifugally separated. Mesoporous tungsten trioxide was obtained by washing the solid with deionized water several times and drying at 150 ℃ for 10 h. The yield was about 80-90%.
(2)Preparation of manganese calcium oxide. Under magnetic stirring, calcium acetate and manganese acetate tetrahydrate were dissolved in deionized water and slowly added to KOH aqueous solution (1-10 mol/L), then brown suspension was obtained. KMnO4 (0.02-0.1 mol/L) aqueous solution was added dropwise. After 5-10 h of continuous stirring reaction, the solid was separated by centrifugation, washed several times by deionized water, dried in vacuum, and then in argon. Calcium manganese oxide was obtained by calcination at 300 ~ 600 ℃ for 2 ~ 8 h in a tubular furnace with atmospheric atmosphere, and the yield was about 90 - 95%.
(3)Preparation of tungstic manganese calcium / mesoporous tungsten oxide composite catalyst. Mesoporous tungsten trioxide and manganese-calcium oxide were added into the ball mill, grinded at room temperature of 300-500 rpm for 3-10 hours on a star mill, dispersed into deionized water, and reacted for 5-20 hours by ultrasonic wave. After centrifugal separation, the solid was dried in vacuum and calcined in a tubular furnace in argon atmosphere for 1-4 hours at 200-600 C, the composite of mesoporous tungsten oxide was obtained. The yield is about 90-98%.
The catalyst composed of mesoporous tungsten trioxide and layered manganese-calcium oxide complex has high photocatalytic activity and stability; the prepared mesoporous tungsten trioxide and layered manganese-calcium oxide form island-like tungsten-manganese-calcium ternary complex on the surface of Mesoporous Tungsten Trioxide by physical grinding, ultrasonic stripping deposition and high temperature calcination. It can be used as a photocatalytic material for preparing photochemical cells. The photocatalytic anode prepared by this composite material has the advantages of good stability, wide photoresponse range, high catalytic activity, simple preparation method, no precious metals and no environmental pollution.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com